Abstract
Purpose
Hip fractures in elderly patients are associated with increased postoperative morbidity and mortality. We evaluated whether a perioperative multi-system optimization protocol can reduce postoperative complications in these patients.
Methods
Immediately after diagnosis of hip fracture, patients ≥ 60 yr were randomized to an intervention or control group. Patients in the intervention group were admitted to our postanesthesia care unit where they were treated with goal-directed hemodynamic management, optimized pain therapy, oxygen therapy, and optimized nutrition. Patients in the control group were managed according to our usual standard of care on a regular ward. Postoperative complications during hospital stay included pre-determined cardiovascular, respiratory, neurologic, renal, or surgical events.
Results
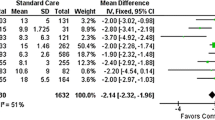
The incidence of at least one postoperative complication (primary outcome) was seen in 32 of 65 (49%) controls compared with 24 of 62 (39%) in the intervention group (relative risk [RR], 0.79; 95% confidence interval [CI], 0.53 to 1.17; P = 0.23). The secondary unadjusted outcomes showed that patients in the intervention group received more Ringer’s acetate compared with controls (median difference, 1.3 L; 95% CI, 0.6 to 2.1 L; P < 0.001), had more frequently a mean arterial pressure > 70 mmHg (57% control vs 75% intervention; median percentage difference, 16%; 95% CI, 7 to 25%; P = 0.001), better pain control (numeric rating scale < 4 at all postoperative measurements; 25% control vs 81% intervention; RR, 0.26; 95% CI, 0.15 to 0.43; P < 0.001), and possibly a lower incidence of acute renal failure (RR, 0.37; 95% CI, 0.14 to 0.98; P = 0.04).
Conclusions
The implementation of a perioperative multi-system optimization protocol algorithm did not significantly reduce the risk of postoperative complications. Nevertheless, we likely over-estimated the potential treatment effect in our study design and thus were under-powered to show an effect.
Trial registration
Clinicaltrials.gov (NCT01673776). Registered 23 August, 2012.
Résumé
Objectif
Chez les patients âgés, les fractures de la hanche sont associées à une augmentation de la morbidité et de la mortalité postopératoires. Nous avons tenté de déterminer si un protocole d’optimisation périopératoire multisystémique pourrait réduire les complications postopératoires chez ces patients.
Méthode
Immédiatement après avoir reçu un diagnostic de fracture de la hanche, les patients ≥ 60 ans ont été randomisés à une intervention ou au groupe témoin. Les patients du groupe intervention ont été admis à notre salle de réveil, où ils ont bénéficié d’une prise en charge avec un traitement à visée hémodynamique, d’un traitement contre la douleur optimisé, d’oxygénothérapie, et d’une nutrition optimisée. Les patients du groupe témoin ont été pris en charge selon notre norme de soins habituelle dans un département de soins courants. Les complications postopératoires pendant le séjour à l’hôpital comprenaient des événements cardiovasculaires, respiratoires, neurologiques, rénaux et chirurgicaux déterminés à l’avance.
Résultats
L’incidence d’au moins une complication postopératoire (critère d’évaluation principal) a été observée chez 32 des 65 (49 %) patients du groupe témoin, par rapport à 24 des 62 (39 %) patients du groupe intervention (risque relatif [RR], 0,79; intervalle de confiance [IC] à 95 %, 0,53 à 1,17; P = 0,23). Les critères secondaires non ajustés ont démontré que les patients du groupe intervention ont reçu plus de solution d’acétate Ringer que les patients du groupe témoin (différence médiane, 1,3 L; IC 95 %, 0,6 à 2,1 L; P < 0,001), que leur tension artérielle moyenne était plus souvent > 70 mmHg (57 % groupe témoin vs 75 % groupe intervention; différence de pourcentage médiane, 16 %; IC 95 %, 7 à 25%; P = 0,001), qu’ils bénéficiaient d’un meilleur contrôle de la douleur (échelle d’évaluation numérique < 4 pour toutes les mesures postopératoires; 25 % groupe témoin vs 81 % groupe intervention; RR, 0,26; IC 95 %, 0,15 à 0,43; P < 0,001), et avaient possiblement une incidence plus faible d’insuffisance rénale aiguë (RR, 0,37; IC 95 %, 0,14 à 0,98; P = 0,04).
Conclusion
La mise en œuvre d’un algorithme de protocole d’optimisation périopératoire multisystémique n’a pas réduit de manière significative le risque de complications postopératoires. Toutefois, nous avons probablement surestimé l’effet potentiel du traitement dans la conception de notre étude, laquelle a par conséquent manqué de puissance pour démontrer un effet.
Enregistrement de l’étude
Clinicaltrials.gov (NCT01673776). Enregistrée le 23 août 2012.
Similar content being viewed by others
The occurrence of a proximal femoral fracture (i.e., hip fracture) is a major medical and surgical concern in older people.1 Compared with matched controls, patient mortality is increased within the first year after a hip fracture,2,3 partly due to specific, and potentially avoidable, postoperative complications.4,5 Therefore, several studies have tried to intervene in the perioperative phase to reduce postoperative complications.6,7 Most studies have focused on only single intraoperative interventions such as optimized pain therapy8 or goal-directed hemodynamic therapy.9,10,11 Meta-analyses, however, question if optimization of pain therapy12 or hemodynamic management13 alone are actually able to improve postoperative morbidity and mortality. In addition, interpretation of these studies is further limited by inadequate statistical power and heterogeneity between studies.
Accordingly, it seems reasonable to combine potentially beneficial interventions over the entire perioperative period into a multi-system optimization protocol regimen to magnify any beneficial effects of the individual interventions. We studied an interventional regimen that included optimized hemodynamic management, intensified pain therapy, maintenance of sufficient oxygenation, and optimal nutrition starting immediately after hip fracture diagnosis. We hypothesized that this combined perioperative multi-system optimization protocol would be able to reduce the incidence of postoperative complications in older patients with hip fractures.
Methods
The study was approved by the local ethics committee (Ethikkommission der Fakultät für Medizin der Technischen Universität München, Ismaninger Str. 22, 81675 München, Germany, Chair: Prof. Dr. G. Schmidt, ID: 5102/11 approved on: 8 September 2011) and was registered at clinicaltrials.gov (Identifier: NCT01673776; registered on 23 August 2012). There was one amendment to the methods, which became effective before the first patient was included (and before the trial was registered). We performed this prospective, randomized, single-centre study at a university hospital in Munich, Germany.
Study population and randomization
We included patients ≥ 60 yr undergoing surgery for hip fracture (femoral neck fracture or trochanteric femoral fracture). Exclusion criteria were pathologic fractures, multiple trauma, and fractures occurring during a hospital stay because of a different disease. After diagnosis of the fracture in the emergency department, a research team member evaluated the patients’ eligibility, informed the patient or their representative in detail about the study, and obtained written informed consent. Patients were allocated to the intervention or control group in a 1:1 ratio. The randomization list was generated by a random generator without blocks (Microsoft Excel for MAC 14.0). For each randomization number, we prepared a paper-based folder with all required materials including the group assignment. Only the folder with the lowest number was accessible.
Multi-system optimization protocol
Our intervention started immediately after inclusion of the patient. It mainly consisted of an intensive pain and hemodynamic optimization protocol, operationalized in patients admitted preoperatively to the postanesthesia care unit (PACU) before surgery, with their care continued postoperatively again in the PACU. The PACU is staffed by one anesthesiologist and three specialist nurses in anesthesia or intensive care that are available to treat nine patients.
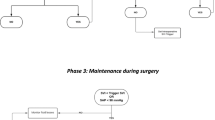
PACU monitoring routinely consists of electrocardiogram (ECG), pulse oximetry, and non-invasive blood pressure. In the intervention group, hemodynamics were additionally optimized using the PulsioFlex® device (PULSION Medical Systems SE; Feldkirchen; Germany) according to a previously published goal-directed hemodynamic protocol (Fig. 1).14 The hemodynamic optimization protocol was also applied intraoperatively.
Hemodynamic treatment algorithm in the perioperative multi-system optimization protocol group. The hemodynamic goals were mean arterial pressure (MAP) > 70 mmHg and a cardiac index (CI) > 2.5 L·kg−1·m−2. Hemodynamics were evaluated routinely every 30 min, as well as at times of hemodynamic instability. We tested fluid responsiveness using a volume challenge of 250 mL Ringer’s acetate. Depending on changes in the stroke volume index (SVI), the patient received either volume or catecholamines in a titrated manner
Pain management was optimized via continuous femoral nerve blockade, which was established immediately after randomization. An initial bolus of 20 mL ropivacaine 0.75% was initially injected followed by a continuous infusion of ropivacaine 0.2% up to 10 mL·hr−1, to reduce pain to a numeric rating scale (NRS) < 4. The NRS presents an 11-point numerical scale, with 0 meaning no pain and 10 the worst imaginable pain.15 In patients whose pain was not sufficiently controlled, we titrated additional boluses of sufentanil (5–10 μg) intraoperatively and piritramid (3–5 mg) postoperatively.
In addition to optimization of hemodynamics and pain, we administered oxygen if the patient’s saturation was < 95% and maintained temperature above 36°C using heating blankets. Patients with a body mass index < 17 kg·m−2 received high caloric (1.5 kcal·mL−1) nutritional drinks twice daily (Fresubin® ENERGY 200 mL; 5.6 g protein; 18.8 g carbohydrates; and 5.8 g fat/100 mL; Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany). The patients stayed in the PACU until surgery.
Control group
We treated control patients according to the standard care of our hospital. After diagnosis, they were transferred to a regular preoperative ward, where routinely four nurses take care of 35 patients. There, routine vital signs (non-invasive blood pressure, heart rate, and pulse oximetry) are obtained every eight hours. The attending surgeon is responsible for pain therapy until transfer to the operating room. This consisted typically of a non-steroidal analgesic such as metamizole or ibuprofen three times a day. In addition, patients received intravenous piritramid if necessary. The control patients were kept fasting until surgery. In case of low blood pressure, the attending surgeons ordered crystalloid therapy at their discretion. Oxygen was applied for oxygen saturations < 90%. Further, patients were not routinely warmed preoperatively and no nutritional drinks were applied. During surgery and postoperatively in the PACU, the attending anesthesiologists administered fluids and catecholamines at their discretion. After discharge from the PACU, all patients were treated according to standard care on a regular postoperative ward, including early mobilization, anticoagulation, and antibiotic therapy.
Anesthesia and surgery
All patients were evaluated for delirium using the confusion assessment method and performed a mini-mental state examination to study cognitive function preoperatively following informed consent in the emergency department.16,17
All patients (intervention and control group) underwent general anesthesia using a combination of sufentanil, propofol, and sevoflurane, and were paralyzed with rocuronium. All patients were intubated and ventilated. In all patients, we monitored vitals intraoperatively according to the hospital’s standard of care including ECG, non-invasive blood pressure, pulse oximetry, and quantitative neuromuscular monitoring by kinemyography. None of the patients received neuraxial anesthesia or other regional blocks except the continuous femoral nerve blockade in the intervention group. The surgical approach was similar between groups.
When the hemoglobin level decreased below 80 g·L−1 or the patient showed signs of ischemia (i.e., electrocardiographic ST-T changes), we administered packed red blood cells. We transfused fresh frozen plasma if the international normalized ratio was > 1.5 or activated partial thromboplastin time was > 45 sec or in case of ongoing bleeding that could not be controlled by surgical interventions.
Outcome
The primary endpoint was the incidence of at least one postoperative complication occurring during the entire hospital stay as determined by a pre-defined set of cardiovascular, respiratory, neurologic, renal, or surgical events. An outcome assessor (who was not blinded to the intervention because of the patient location) examined the patients on days 1 and 2 after surgery. During these visits, the outcome assessor identified delirium using the confusion assessment method and by interviewing the nurses and relatives. Further, pain was determined with the NRS. The pre-specified complications as listed in Table 1 were analyzed by physical examination of the patients as well as by review of the patients’ electronical and written medical records. On the day of hospital discharge, a mini-mental state examination was performed and again the patients’ records were reviewed to identify complications during the entire hospital stay.16 Further, in-hospital mortality, and intensive care unit (ICU) and hospital length of stay were recorded.
Patients were subsequently followed for up to 12 months after surgery via telephone interview to assess mortality. In cases where we were not able to reach the patient, we reviewed the hospital record for information about survival during the last year.
To compare intervention and control patients, we also calculated the success rate in the achievement of the targeted multi-system optimization protocol goals. Every measured mean arterial pressure (MAP), NRS, oxygen saturation, and cardiac index was assessed accordingly to its respective target with success rates given as the percentage of values within the targets of all measured values.
Statistical analysis
We based our sample size calculation on data from patients who underwent surgery after femoral neck fracture during 2009 in our hospital. During this period, 50% of the patients developed at least one postoperative complication (as listed in Table 1). Assuming a 50% relative reduction of the incidence of complications, along with 80% power and a two-sided P < 0.05, we calculated a sample size of 66 patients per group would be needed.
Outcomes are given as numbers (percentages). They were analyzed with Chi square tests. Analyses are presented as relative risk (RR) and the corresponding 95% confidence interval (CI).18 Continuous variables including the achievement rates are given as median (interquartile range). They were analyzed by Mann–Whitney U tests. Their median differences and 95% CIs were calculated according to Lehmann–Hodges.19 The significance level for the primary outcome was P < 0.05. Secondary endpoints were not adjusted for multiple comparisons. We performed the analyses with SPSS Statistics® (Version 24.0; IBM; NY, USA).
Results
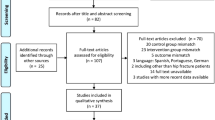
Patients were enrolled from 12 March 2012 to 11 November 2015 and finished the one-year follow up in October 2016. Figure 2 shows the CONSORT diagram of the study. We analyzed 65 patients in the control group and 62 in the intervention group. Baseline characteristics were comparable between the two groups regarding type of fracture or surgery, pre-medical condition, and renal function (Table 2).
The incidence of at least one postoperative complication (primary outcome) was not significantly different, occurring in 39% of patients in the intervention group compared with 49% of patients in the control group (RR, 0.79; 95% CI, 0.53 to 1.17; P = 0.23). Analysis of specific complications revealed a possibly reduced incidence of acute renal failure in patients receiving the perioperative multi-system optimization protocol (P = 0.04). In-hospital mortality and duration of ICU stay and hospital stay were not different between groups (P = 0.96, P = 0.28, P = 0.64, respectively). Data on one-year mortality were available for 122 of the 127 patients investigated (96%) and did not differ between the two groups (P = 0.68) (Table 3).
Patients in the intervention group spent more time in the PACU treated by an anesthesiologist before and after surgery. They received more Ringer’s acetate, whereas more patients in the control group were treated with colloids (Table 4). In the preoperative period, there was no patient in the intervention group with a MAP < 70 mmHg (median achievement rate, 100%). During surgery, a median of 75% patients in the intervention group achieved a MAP > 70 mmHg compared with 57% of the control patients (median percentage difference, 16%; 95% CI, 7 to 25%; P = 0.001). A cardiac index > 2.5 L·min−1 was measured in the intervention group preoperatively in 100% of all measurements, intraoperatively in 40%, and postoperatively in 100%.
Intraoperatively, 59 of 65 patients (91%) in the control group and 58 of 62 patients (94%) in the intervention group received noradrenaline infusions (P = 0.56) in similar doses, while seven of 65 (11%) patients in the intervention group and no control group patient received dobutamine (P = 0.009). Postoperatively, two of 62 patients (3%) in the intervention group and five of 65 control patients (8%) still needed noradrenaline infusions (P = 0.20).
Nine of 62 patients (15%) in the intervention group were transfused with red packed cells (one to seven units) and two of 62 (3%) with fresh frozen plasma (two to four units). Nine of 65 patients (14%) in the control group received red packed cells (one to four units) and three of 65 (5%) fresh frozen plasma (two units each) (P = 0.91 and P = 0.69).
Every patient in the control group had an oxygen saturation ≥ 95% during anesthesia. Each patient in the intervention group had an oxygen saturation ≥ 95% in the preoperative period and during anesthesia. In the postoperative period, there were comparably high achievement rates of oxygen saturation ≥ 95% in both groups (P = 0.80).
Patients in the intervention group had lower absolute pain levels postoperatively (Fig. 3). At all postoperative measurements, 50 of 62 patients (81%) in the intervention group and 16 of 65 patients (25%) in the control group had an NRS < 4 (RR, 0.26; 95% CI, 0.15 to 0.43; P < 0.001).
A) Mean arterial pressure (MAP) pre-induction, during surgery, and in the postanesthesia care unit (PACU). During surgery: median difference, 3 mmHg; 95% confidence interval, 1 to 7 mmHg; P = 0.018. B) Numeric rating scale (NRS) preoperative, in the postanesthesia care unit (PACU) and on postoperative days one and two. Data are depicted using box plots of the control and the intervention patients (perioperative multi-system optimization protocol). The horizontal lines within the boxes indicate the group medians, the boxes depict the interquartile ranges [IQR], the whiskers indicate the farthest value outside the boxes limited to 1.5 of the respective IQR, further outliers are plotted as individual points. At PACU: median difference, -3; 95% confidence interval, − 3 to − 2; P < 0.001; on day 1: − 2; − 2 to − 1; P < 0.001; on day 2: − 2; − 2 to − 1; P < 0.001. *P = 0.018 intervention vs control, †P < 0.001 intervention vs control
Discussion
In this single-centre, unblinded, randomized-controlled trial, we did not find a decrease in complications in older hip fracture patients who were treated perioperatively with a complex optimization protocol compared with standard care. Nevertheless, the protocol did result in lower pain scores, higher MAPs, and possibly decreased the incidence of mild acute kidney injury (all secondary endpoints). Nevertheless, the data of this monocentric trial do not support routine use of such a complex perioperative intervention.
This study was designed to show that a perioperative multi-system optimization protocol could reduce the number of patients with at least one postoperative complication by 50%. Based on this assumption, 66 patients were needed to confirm our hypothesis. Since the protocol, however, reduced the incidence of any complication from 49% to 39% (i.e., a RR reduction by 21%), we failed to show the anticipated 50% reduction in postoperative complications. The effect of this intervention, however, is in line with the investigations by Bartha et al. and Moppett et al., as well as a recent Cochrane review.9,11,13 Those authors also implemented a goal-directed hemodynamic algorithm and reported a trend to a reduced risk in their intervention groups only.
To confirm a 20% RR reduction using our protocol, we would require 400 patients per group to achieve 80% power. The multi-system optimization protocol used herein required the allocation of systemic as well as personnel resources, including during night shifts, mainly related to PACU capacity, and an additional anesthesiologist (who in our study was a member of the study team). This resulted in an elaborate and complex approach. Nevertheless, we assume that this could be feasibly applied in a multicentre exercise.
Many studies investigating the effects of goal-directed therapy protocols including our own work showed comparable achievement rates between treatment groups.20 If adding little beyond standard care to achieve the target values, any protocol or algorithm is unlikely to reduce complications.9 This may be due to either an overall good standard of care or a minor preexisting impaired specific target function. Not all patients with hip fracture have the same impairment but almost every patient has at least one preoperative problem. Accordingly, we expected that a bundle of measures improves the physical status of all patients and is more likely to reduce postoperative complications. Our perioperative multi-system optimization protocol provided a MAP that was more frequent in the target range and pain scores that were more likely to be under control (NRS < 4). Obviously, these effects were not enough to significantly reduce overall postoperative complications.
The intervention was associated with an elevated amount of fluids infused before and after surgery. The higher amount of Ringer’s acetate resulted in better intraoperative hemodynamic stability in the intervention group. Therefore, an optimized renal perfusion most likely contributed to the marginally improved postoperative renal function concerning Acute Kidney Injury Network stages 1 and 2 only. Important to note, we did not see an increased rate of pulmonary complications. In control patients, hypotension and hypovolemia probably provoked the higher incidence of renal failure.21 Nevertheless, more control patients also received colloids. The previously described nephrotoxic effect of fluid resuscitation with colloids might have additionally impaired renal function in these patients.22,23
There were several other limitations to our study. One is related to the timing of the patients’ admission and the subsequent surgery. Delayed surgery of proximal femoral neck fracture is associated with higher mortality, particularly if it is postponed for more than 24 hr.24,25,26 The preoperative initiation of a multi-system optimization protocol in the anesthesiologists’ facility included the risk of further delay of surgery. Nevertheless, the time interval between admission to the hospital and surgery was lower than 24 hr and comparable between groups. Thus, although all patients had surgery without unnecessary delays, there may also have been inadequate time to more fully benefit from the preoperative optimization that was being studied.
Another limitation of our study is that the patients and the outcome assessors were not blinded. This was because of the PACU location for intervention group patients who also had a femoral nerve catheter, which was accessible during the physical examination during the postoperative visits. Nevertheless, a lack of blinding commonly confers the risk of favouring the intervention group implicitly or explicitly. Our study, however, reports negative results for the investigational protocol.
A major difference between our standard of care and that of other hospitals may be our physician-based PACU. Every patient is transferred postoperatively to the PACU and stays there for up to 18 hr. One nurse takes care of at most three PACU patients, and one anesthesiologist is responsible for at most nine PACU patients. This prolonged PACU approach to care may have benefited our patients’ outcomes in both groups allowing the treatment of any pathology earlier before it became severe.
The multi-system optimization protocol included peripheral nerve blockade because it allows long-lasting pain control.27 Therefore, we did not allow neuraxial anesthesia in any group. This can be considered a limitation of our study. Nevertheless, if not applied continuously, neuraxial anesthesia has only a temporary effect on pain. Furthermore, there is neither a reduction in postoperative delirium rate,28 nor reduced mortality after spinal anesthesia.29
In conclusion, implementation of a multi-system optimization protocol did not significantly reduce the risk of postoperative complications as hypothesized. Secondary analyses suggest improvements in pain control and marginally improved rates of mild acute kidney injury. A larger, multicentre, randomized clinical trial recruiting at least 800 patients would be necessary to determine whether the marginal advantages we showed justify the large (and costly) efforts of our perioperative multi-system optimization protocol.
References
Heinrich S, Rapp K, Rissmann U, Becker C, Konig HH. Service use and costs of incident femoral fractures in nursing home residents in Germany: the Bavarian Fall and Fracture Prevention Project (BF2P2). J Am Med Dir Assoc 2011; 12: 459-66.
Giversen IM. Time trends of mortality after first hip fractures. Osteoporos Int 2007; 18: 721-32.
Haentjens P, Magaziner J, Colon-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 2010; 152: 380-90.
Roche JJ, Wenn RT, Sahota O, Moran CG. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ 2005. DOI:https://doi.org/10.1136/bmj.38643.663843.55.
Ali AM, Gibbons CE. Predictors of 30-day hospital readmission after hip fracture: a systematic review. Injury 2017; 48: 243-52.
Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma 2014; 28: e49-55.
Prestmo A, Hagen G, Sletvold O, et al. Comprehensive geriatric care for patients with hip fractures: a prospective, randomised, controlled trial. Lancet 2015; 385: 1623-33.
Kang H, Ha YC, Kim JY, Woo YC, Lee JS, Jang EC. Effectiveness of multimodal pain management after bipolar hemiarthroplasty for hip fracture: a randomized, controlled study. J Bone Joint Surg Am 2013; 95: 291-6.
Bartha E, Arfwedson C, Imnell A, Fernlund ME, Andersson LE, Kalman S. Randomized controlled trial of goal-directed haemodynamic treatment in patients with proximal femoral fracture. Br J Anaesth 2013; 110: 545-53.
Moppett IK, White S, Griffiths R, Buggy D. Tight intra-operative blood pressure control versus standard care for patients undergoing hip fracture repair - Hip Fracture Intervention Study for Prevention of Hypotension (HIP-HOP) trial: study protocol for a randomised controlled trial. Trials 2017. DOI:https://doi.org/10.1186/s13063-017-2066-5.
Moppett IK, Rowlands M, Mannings A, Moran CG, Wiles MD, NOTTS Investigators. LiDCO-based fluid management in patients undergoing hip fracture surgery under spinal anaesthesia: a randomized trial and systematic review. Br J Anaesth 2015; 114: 444-59.
Guay J, Parker MJ, Griffiths R, Kopp S. Peripheral nerve blocks for hip fractures. Cochrane Database Syst Rev 2017; 5: CD001159.
Lewis SR, Butler AR, Brammar A, Nicholson A, Smith AF. Perioperative fluid volume optimization following proximal femoral fracture. Cochrane Database Syst Rev 2016; 3: CD003004.
Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445]. Crit Care 2005; 9: R687-93.
Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth 2011; 107: 619-26.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189-98.
Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990; 113: 941-8.
Katz D, Baptista J, Azen SP, Pike MC. Obtaining confidence intervals for the risk ratio in cohort studies. Biometrics 1978; 34: 469-74.
Hodges J, Lehmann E. Estimates of location based on rank tests. Ann Math Stat 1963; 34: 598-611.
Schmid S, Kapfer B, Heim M, et al. Algorithm-guided goal-directed haemodynamic therapy does not improve renal function after major abdominal surgery compared to good standard clinical care: a prospective randomised trial. Crit Care 2016. DOI:https://doi.org/10.1186/s13054-016-1237-1.
Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev 2015; 95: 405-511.
Moeller C, Fleischmann C, Thomas-Rueddel D, et al. How safe is gelatin? A systematic review and meta-analysis of gelatin-containing plasma expanders vs crystalloids and albumin. J Crit Care 2016; 35: 75-83.
Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA 2013; 309: 678-88.
Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anesth 2008; 55: 146-54.
Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am 2015; 97: 1333-9.
Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA 2017; 318: 1994-2003.
Henderson CY, Abdel-Galil R, Woo MY, et al. Improving care for elderly patients with hip fracture: interdisciplinary collaboration in regional analgesia. Can J Anesth 2019; 66: 845-6.
Zhang H, Lu Y, Liu M, et al. Strategies for prevention of postoperative delirium: a systematic review and meta-analysis of randomized trials. Crit Care 2013. DOI:https://doi.org/10.1186/cc12566.
White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia 2014; 69: 224-30.
Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007. DOI:https://doi.org/10.1186/cc5713.
Acknowledgements
We are indebted to all doctors and nurses looking after the patients in the operation theatre and at the postanesthesia care unit.
Conflict of interests
The authors declare that they have no competing interests.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Sebastian Schmid and Bettina Jungwirth were involved in the study design, patient recruitment, data collection, data analysis, and writing of the manuscript. Manfred Blobner was involved in the study design and data analysis, and gave critical feedback on the manuscript. Brigitte Haas was involved in patient recruitment and data collection, and gave critical feedback on the manuscript; Martin Lucke and Markus Neumaier were involved in patient recruitment and gave critical feedback on the manuscript; Aida Anetsberger was involved in study design, patient recruitment and data collection, and gave critical feedback on the manuscript.
Funding
This study was funded by institutional support. There was no involvement of any sponsor in study design, collection, analysis, and interpretation of data, writing of the report, and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schmid, S., Blobner, M., Haas, B. et al. Perioperative multi-system optimization protocol in elderly hip fracture patients: a randomized-controlled trial. Can J Anesth/J Can Anesth 66, 1472–1482 (2019). https://doi.org/10.1007/s12630-019-01475-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-019-01475-9