Abstract
Carfentanil is a synthetic fentanyl analogue approved for veterinary use. It is a mu-opioid receptor agonist with an estimated analgesic potency approximately 10,000 times that of morphine and 20-30 times that of fentanyl, based on animal studies. Since 2016, an increasing number of reports describe detection of carfentanil in the illicit drug supply. Little is known about the pharmacology of carfentanil in humans. Its high potency and presumed high lipophilicity, large volume of distribution, and potential active metabolites have raised concerns about the management of people exposed to carfentanil as well as the safety of first responders. Exposed individuals exhibit features of an opioid toxidrome and respond to opioid antagonists such as naloxone, although empiric dose requirements are unknown and very high doses may be required. Rare reports of suspected accidental poisoning of first responders have not been analytically confirmed and are unlikely to represent true poisoning. General occupational hygiene measures, including regular decontamination with soap and water, basic personal protective equipment (nitrile gloves, N95 mask, and eye goggles), and ready access to naloxone are generally sufficient in most circumstances.
Résumé
Le carfentanil est un analogue synthétique du fentanyl approuvé pour usage vétérinaire. C’est un agoniste du récepteur mû des opioïdes ayant une puissance analgésique estimée, à partir d’études chez l’animal, à environ 10 000 fois celle de la morphine et 20 à 30 fois celle du fentanyl. Depuis 2016, un nombre croissant de rapports font état de la présence de carfentanil dans les drogues illicites. On ne sait que peu de choses sur la pharmacologie du carfentanil chez l’homme. Sa puissance pharmacologique et sa forte lipophilie supposée, un grand volume de distribution et de possibles métabolites actifs ont soulevé des préoccupations pour le traitement des personnes exposées au carfentanyl et pour la sécurité des premiers intervenants. Les sujets exposés présentent les caractéristiques d’un syndrome toxique aux opioïdes et répondent à leurs antagonistes, comme la naloxone, bien que les doses empiriques nécessaires soient inconnues et qu’il faille possiblement administrer de très fortes doses. Les rares descriptions d’intoxications accidentelles suspectées chez des premiers intervenants n’ont pas été confirmées par des analyses et il est peu probable qu’elles représentent de véritables empoisonnements. Des mesures générales d’hygiène professionnelles, incluant une décontamination régulière à l’eau et au savon, un équipement de protection individuelle élémentaire (gants en nitrile, masque N95, et lunettes de protection), ainsi qu’un accès rapide à la naloxone sont généralement suffisants dans la majorité des cas.
Similar content being viewed by others
Epidemiology
Fentanyl and its many analogues, including carfentanil, have become a major public health concern in recent years. They are responsible for an ever-increasing number of opioid-related overdoses and deaths in North America1,2 and Europe. In 2016, fentanyl and its analogues contributed to at least 1,516 deaths in Canada.3 Preliminary data from 2017 suggest these agents continue to play an increasing role in opioid-related deaths.3
In the United States, carfentanil was first recognized as an additive to other abused drugs in mid-2016.4,5 Similarly, law enforcement reports of carfentanil poisoning and seizures first appeared in Canada in 2016.6,7,8,9,10 In 2016, 22 of 343 overdose deaths involving fentanyl in Alberta were reported to have involved carfentanil.11 Nevertheless, the true number of deaths due to carfentanil is probably higher because of limitations in identifying non-fentanyl synthetic opioids such as carfentanil.
Most fentanyl analogues, including carfentanil, are thought to be imported into North America from abroad, notably China, typically in powder form or in counterfeit tablets resembling legitimate pharmaceutical products. These analogues may also be used to adulterate illicit opioids or other drugs.2 In June 2016, the Royal Canadian Mounted Police seized 1 kg of carfentanil shipped from China to Vancouver disguised as printer ink cartridges.12 On March 1, 2017, political pressure led China’s National Narcotics Control Commission to add carfentanil, along with several other fentanyl analogues, to its list of controlled substances.13 Prior to this, these agents were not regulated.
Background
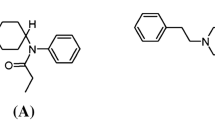
Carfentanil (International Union of Pure and Applied Chemistry name: 4-[{1-oxopropyl}-phenyl- amino]-1-[2-phenylethyl]-4-piperidinecarboxylic acid methyl ester) is an analogue of the synthetic opioid fentanyl (Figure). It was first synthesized by Janssen Pharmaceuticals (Beerse, Belgium) in 1974 under the brand name Wildnil® and approved in 1986 for veterinary use as an intramuscular tranquilizer for large animals.14 To receive authorization for its use in the United States, veterinarians must be on an “Approved Carfentanil Users List” and have special Drug Enforcement Agency (DEA) registration class approval.15 Commercial production of Wildnil ceased in 2003, and carfentanil is now only available in compounded 10 mL vials of 3 mg·mL−1. In Canada, it is a Schedule I substanceFootnote 1 under the Controlled Drugs and Substances Act.16 It is not approved for human use except in the research setting as radiolabelled [11C]-carfentanil, where it is used to map mu-opioid receptors in humans by positron emission tomography at doses lower than 7 µg.17
Pharmacokinetics
Pharmacokinetic information is limited to animal and in vitro studies as well as a few scattered case reports of intentional or unintentional human exposures.
Approved routes of administration in animals include intramuscular, intravenous, transmucosal, and oral.14 Most human exposure to carfentanil occurs through intake of adulterated heroin or fentanyl, therefore human routes of carfentanil administration are similar to those of heroin or fentanyl. These include oral ingestion via counterfeit tablets (most common) as well as intravenous and nasal insufflation routes.20
It is inferred that carfentanil is highly protein bound, owing to its structural similarity to fentanyl and sufentanil, which exhibit protein binding of 84.4% and 92.5%, respectively.21 In one study, low-dose radiolabelled [C-11] carfentanil was administered intravenously to healthy controls at an average dose of 1.34 µg (0.019 mg·kg−1).22 The mean (standard deviation) elimination half-life was 41.8 (17.5) min. A recent case report of intentional carfentanil exposure measured carfentanil and norcarfentanil levels over 52 hr.23 The respective half-lives were calculated as 5.7 hr and 11.8 hr using a one-compartment open pharmacokinetic model.23 There was no evidence of saturation pharmacokinetics. The former study limited blood sampling to 90 min and may not have accurately characterized the true elimination half-life, whereas the latter is likely more representative of the distributional half-life of carfentanil.22
Carfentanil has a calculated logP value of 3.7, indicating high lipophilicity.24 From this, and by analogy with other structurally similar drugs, it can be inferred that carfentanil distributes preferentially throughout extravascular compartments, including the brain and adipose tissue. The phenomena of “re-narcotization”—i.e., the recurrence of opioid toxicity up to 72 hr after apparent recovery—has been observed in large animals tranquilized with carfentanil.25 Possible mechanisms for this include release of agonist from body depots after its antagonist has been eliminated, generation of active metabolite(s), under-dosing of the mu-opioid receptor antagonist, and preferentially rapid metabolism of the antagonist relative to the agonist.18 Lipophilicity and protein binding are presumed to contribute to carfentanil’s large distribution volume and re-entry of carfentanil from sequestered compartments is highly plausible.26,27 A similar delayed respiratory depression has also been observed in humans after high-dose fentanyl anesthesia.28
The hepatic cytochrome P450 enzyme system rapidly metabolizes carfentanil.21 While the specific P450 isoforms responsible are not known, CYP3A4 is the most likely isoform based upon analogy of carfentanil with fentanyl.29 An in vitro study of human liver microsomes incubated with carfentanil observed two predominant phase I metabolism pathways: piperidine ring monohydroxylation (C24H30N2O4) and N-dealkylation forming norcarfentanil (C16H22N2O3). The metabolite formed from piperidine ring monohydroxylation appears different to carfentanil.21 In contrast, both carfentanil and remifentanil metabolism can generate norcarfentanil; in humans, metabolism of remifentanil to norcarfentanil is not considered a significant metabolic pathway.30 This observation suggests that identification of the piperidine ring monohydroxylation metabolite may identify carfentanil exposures; nevertheless, it is unclear whether these results can be generalized to in vivo human metabolism. The major phase II metabolite is a glucuronide conjugate of hydroxylated carfentanil.21 A similar study, which incubated human hepatocytes with carfentanil, revealed that the clearance of carfentanil was slower and had a longer half-life compared with human liver microsomes.21 This was attributed to carfentanil’s lipophilicity and/or extensive protein binding.21 Feasel et al. postulated that if carfentanil is highly lipophilic and can easily distribute into cellular membranes with slow liberation, or both, then less carfentanil would be available for hepatic metabolism and extraction.21 Clinically, these properties of carfentanil may contribute to prolonged exposure and drug effect and may explain re-narcotization in animals.
In animals, carfentanil is eliminated through bile and urine.14 These elimination routes are likely the same in humans.21,31 The properties that contribute to carfentanil’s toxicity include its high mu-opioid receptor affinity, lipophilicity, presumed large volume of distribution, and possible active metabolites, specifically those that retain their phenethyl structure.
Pharmacodynamics
Analgesic potency
Based on rat tail withdrawal studies, the relative analgesic potency of carfentanil is approximately 10,000 times that of morphine, 4,000 times that of heroin, and 20-30 times that of fentanyl.32 Ligand receptor binding studies report that the calculated equilibrium dissociation constant (Ki) in inhibitory studies for human opioid receptors are 0.024 nM (μ1), 3.3 nM (δ), and 43 nM (κ). In comparison, Ki values for fentanyl were 1.9 nM (μ1), 153 nM (δ), and 197 nM (κ).33 This suggests that carfentanil has a higher affinity for the aforementioned receptors relative to fentanyl.
Therapeutic index
Calculated as the median lethal dose (LD50)/lowest median effective dose (ED50), a high therapeutic index generally implies a wide safety margin between the effective dose and the lethal dose. The reported therapeutic index of carfentanil is 10,600 compared with fentanyl’s 300, using morphine as the reference at 70.34 Nevertheless, this parameter is potentially misleading as it suggests that carfentanil is safer than fentanyl, even though the reported LD50 values in rats are similar (3.39 mg·kg−1 for carfentanil and 3.05 mg·kg−1 for fentanyl).18,35 The discrepancy between analgesic potency and lethality may be related to the fact that these values are derived from animal studies, and may not be generalizable to other species such as humans.
Exposure diagnosis
As with intoxication with any mu-opioid receptor agonist, individuals with systemic carfentanil exposure show features typical of an opioid toxidrome, including miosis, respiratory depression, and altered mental status. Not all features may be present if there are co-ingestions. Untreated, anoxic brain injury can lead to death. Although not yet reported with carfentanil, chest wall rigidity seen with intravenous fentanyl use may also be a significant and unreported factor contributing to rapid mortality.36 Chest wall rigidity has also been reported following high doses and rapid administration of other lipophilic synthetic opioids, such as alfentanil, sufentanil, and remifentanil.37
The first confirmed human carfentanil exposure involved a 42-yr-old veterinarian who accidently splashed a dart containing carfentanil citrate 1.5 mg and xylazine 50 mg into his eyes and mouth while attempting to dislodge the dart from a tree. Despite copious irrigation, he developed drowsiness within two minutes and was administered naltrexone 100 mg parenterally (supplied with the carfentanil in the same package) in the pre-hospital setting. He was discharged 24 hr later and recovered completely.38
Carfentanil was implicated as one of the immobilizing agents used by the Russian Special Forces during the October 2002 Nord-Ost terrorist attack in a Moscow theatre. Fifteen minutes prior to Special Forces infiltration of the theatre, an aerosol was introduced through the ventilation system causing hundreds of people, including hostages, to exhibit opioid toxicity. Due to the delay in identifying the immobilizing agent and inadequate healthcare resources available to manage the crisis, 127 of the 800 hostages died and more than 650 were hospitalized. Although the Russian Health Minister identified the aerosol as an unspecified fentanyl derivative, liquid chromatography-tandem mass spectrometry (LC/MS/MS) analysis of clothing extracts from two survivors, and urine from a third survivor, confirmed the presence of norcarfentanil (a metabolite of both carfentanil and remifentanil) and urinary methyl-4-((propionyl)phenylamino)piperidine-4-carboxylate (a N-dealkylation product of carfentanil and remifentanil).30
Exposure detection
Many unintentional exposures to carfentanil through adulterated heroin have been documented in North America39 and Europe.40 These reports are almost certainly an underestimation of the true prevalence of carfentanil exposure. Standard screening with gas chromatography mass spectrophotometry (GC/MS) is often unable to detect very small quantities of carfentanil because of the instrument’s detection limits.39,41 Given that carfentanil is more potent than other opioids, the sensitivity of instrumentation to detect minute quantities is critical. In one series of synthetic opioid deaths, 134 carfentanil-related deaths were detected using liquid chromatography (LC)-ion trap MS, whereas GC/MS missed 100 of these cases. The reported detection limits were ten-fold different (0.1 ng·mL−1 and 1 ng·mL−1, respectively).39 Other analytical methods have also been documented,18 including high pressure LC-atmospheric pressure ionization tandem mass spectrometry (LC/MS/MS), with even more sensitive limits of detection of 3 pg·mL−1 and 27 pg·mL−1 respectively.42
Additional factors contributing to underestimation of carfentanil exposure are the lack of widespread availability of sufficiently sensitive instrumentation such as LC/MS/MS to perform these targeted analyses and/or failure to perform analyses when toxicologically significant levels of other drugs are found.41 Even when instrumentation is available, toxicology laboratories must obtain analytical standards of parent compounds (and metabolites, if known) to identify unknowns.43 These are often not commercially available because of their legal status, and bureaucratic challenges with import requests or Health Canada approval may cause additional delays.43
Recreational use
Even more disturbing are the emerging reports of deliberate recreational use of carfentanil. One case report documented a 16-yr-old male who presented unconscious, tachycardic, hypotensive, and hypoxic but with normal pupils.31 He also used atomoxetine (a norepinephrine reuptake inhibitor) approved for Asperger’s syndrome. Using GC/MS and LC/MS/MS, he was found to have a serum concentration of 0.6 ng·mL−1 carfentanil and 0.2 ng·mL−1 of norcarfentanil reportedly one hour after use by an unknown route. The corresponding urine concentrations were 1.3 ng·mL−1 and 0.5 ng·mL−1 respectively. He was treated with an unspecified amount of naloxone and recovered.31 Another case used high pressure LC/MS/MS to measure a threshold of wakefulness following carfentanil exposure to be 0.52 ng·mL−1; the lowest limit of carfentanil in the blood that could be detected by LC/MS/MS was 0.2 ng·mL−1.23
Management of carfentanil exposure
Management of synthetic opioid toxicity involves standard resuscitative measures of oxygenation, ventilation, and administration of an opioid antagonist as the main priorities. Naloxone is the preferred competitive mu-opioid receptor antagonist for reversing the effects of carfentanil. It is readily available and can be given intravenously, intranasally (IN), or intramuscularly to reverse opioid-related respiratory depression.44 The onset of action depends on the route of administration but it is often less than five minutes.44 Carfentanil’s high binding affinity and potential for re-narcotization may necessitate repeat dosing of an opioid antagonist. Based on the predicted potency of carfentanil, it has been suggested that the initial naloxone dose administered should be greater than the maximum empiric naloxone dose recommended by the American Heart Association (0.4 mg iv/im or 2 mg IN).45 Nevertheless, this empiric dose is predicted based on binding kinetics and has not been studied, with the exception of a few anecdotal reports, and is unknowable a priori in most individual cases given the uncertainty regarding the dose of carfentanil received. The effective dose of naloxone is empiric and should be dosed to a clinical endpoint of restored ventilation, as was the case in a report of carfentanil exposure of unknown dose. Here, two 2 mg parenteral naloxone doses of unclear timing were administered with no effect, and supportive mechanical ventilation was subsequently provided for 31 hr with no residual sequelae.23
In an observational study of fentanyl reversal in the Cook County Emergency Department, naloxone doses sufficient to reverse respiratory depression ranged from 0.4–12 mg (average = 3.36 mg).46 Of the 15 patient encounters, 15% reversed with a dose of 0.4 mg, and doses > 6mg were required in six instances. This suggests that in a majority of cases, excessive naloxone administration may be unnecessary. This may also be true for carfentanil as animal studies suggest that the relative lethality of carfentanil compared with fentanyl is equivalent.47 In fact, a recent position statement by the American College of Medical Toxicology (ACMT) and American Academy of Clinical Toxicology (AACT) suggests that doses of naloxone beyond 10 mg are unlikely to be helpful.47 Furthermore, 13 µg·kg−1 of naloxone (equivalent to 1 mg of naloxone administered to an 80 kg male) is sufficient to occupy 50% of brain mu-opioid receptors and is often enough to restore respiratory drive.48 If the probability of carfentanil exposure is high, a reasonable starting dose is 2 mg iv.20 Naloxone may be repeated every three to five minutes until respiratory depression reverses or level of consciousness improves. A naloxone infusion may also be required. After reversal requiring higher than 0.4 mg naloxone, extended observation is recommended.49
In circumstances where naloxone is not available, naltrexone is an option.38 Naltrexone can be administered orally, intramuscularly, or intravenously. It has a large distribution volume and has a biphasic peak of effect at two hours and two to three days. For animal reversal, the recommended dosing ratio is 100 mg naltrexone for every 1 mg carfentanil. Re-narcotization after naltrexone administration has been reported in animals given naltrexone doses using this approach.26,27 It is unclear whether this occurs in humans.
Other standard measures include supportive care (airway protection and basic and advanced life support with cardiopulmonary resuscitation if cardiac arrest occurs),14,15 transportation of patients to a hospital setting, and ensuring pre-hospital and hospital opioid antagonist resources are adequate.
Provider safety
The issue of first responder safety in the setting of potent fentanyl analogues such as carfentanil is a concern to many. This is largely based on accounts of law enforcement officials experiencing non-specific symptoms after casual or topical exposure to “white powder” suspected of being a clandestinely produced opioid.50,51 These reports are not consistent with opioid toxicity, and the risk of clinically significant exposure from passive contact with opioids is extremely low.49 Realistically, dermal exposure in the absence of moisture is unlikely to cause an overdose. Contact with mucous membranes via inhalation, eye exposure, or oral exposure has a higher risk; nevertheless, the exposure would have to be prolonged and the dose significant to cause an overdose.47
Basic level personal protective equipment (PPE) most commonly consists of nitrile gloves, safety glasses, N95 mask, disposable paper suit or coveralls, and shoe covers.52 Depending on the potential degree of exposure, level A, B, or C PPE consisting of full chemical resistant suits and a respirator or breathing apparatus can be worn. General occupational hygiene measures are recommended, including routine decontamination immediately after exposure. Hands should be washed with soap and water before and after duties. Hand sanitizer should be avoided as the alcohol may promote transdermal absorption.52 Those with suspected inhalational exposure should be moved to a well-ventilated area and observed for symptoms of opioid toxicity. If toxicity is suspected, empiric administration of naloxone is reasonable and medical assessment can always be considered. Eating, drinking, or smoking in the presence of any suspected fentanyl or fentanyl analogues should be avoided.52,53
The ACMT and AACT state that nitrile gloves and an N95 respirator provide sufficient protection for potential dermal and respiratory exposures.47 Further emphasis is placed on training workers to recognize opioid intoxication and a ready access to naloxone, including the ability to administer it if there are clear objective signs of hypoventilation or a depressed level of consciousness. These are consistent with DEA52 and National Institute for Occupational Safety and Health53 recommendations for first responders, which include maintenance of an individual basic PPE kit that also includes a naloxone injector(s). These safety measures should be in place prior to taking any samples or disturbing any unknown powders. Field testing should be done only with appropriate PPE, which should be disposed of safely. The risk of unmasked violent behavior or sympathetic hyperactivity in patients with stimulant co-ingestions is also important to recognize.
Conclusion
Carfentanil is a highly potent mu-opioid receptor agonist, but in animals its LD50 is not appreciably different from that of fentanyl. The number of true carfentanil human exposures is likely underestimated due to limitations in its routine detection. Although human studies are lacking, carfentanil’s chemical properties are similar to those of other fentanyl analogues. Signs and symptoms characteristic of opioid intoxication are expected following systemic exposure. In addition to basic supportive care and airway management, administration of opioid antagonists, such as naloxone, is critical. Empiric dosing is situation dependent; nevertheless, a starting dose of 2 mg iv is reasonable with continued monitoring for the need of repeat dosing or infusion. Incidental contact with carfentanil is not expected to cause opioid toxicity. Nevertheless, routine precautions, including appropriate occupational hygiene, PPE (nitrile gloves, N95 mask, and eye goggles) and ready access to naloxone, in conjunction with standard supportive care, should be sufficient for the majority of exposures.
Change history
25 February 2019
A Correction to this paper has been published: https://doi.org/10.1007/s12630-019-01310-1
Notes
Under the Controlled Drugs and Substances Act of Canada, a Schedule I substance is defined as a substance with no currently accepted medical use and a high potential for abuse.
References
Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep 2016; 65: 1445-52.
Canadian Centre on Substance Abuse. CCENDU Bulletin: Deaths Involving Fentanyl in Canada, 2009-2014. Available from URL: http://www.ccsa.ca/Resource%20Library/CCSA-CCENDU-Fentanyl-Deaths-Canada-Bulletin-2015-en.pdf (accessed August 2018).
Special Advisory Committee on the Epidemic of Opioid Overdoses. National report: Apparent opioid-related deaths in Canada (January 2016 to June 2017) Web-based Report. Web-based Report. Ottawa: Public Heal Agency Canada; 2017. Available from URL: https://www.canada.ca/en/public-health/services/publications/healthy-living/apparent-opioid-related-deaths-report-2016-2017-december.html (accessed August 2018).
Rotuno-Johnson M. Ohio has most carfentanil seizures in United States. Associated Press, November 3, 2016. Available from URL: http://nbc4i.com/2016/11/03/ohio-has-most-carfentanil-seziures-in-united-states/ (accessed August 2018).
Franko K. A new threat in fight against overdoses: it’s not heroin or fentanyl, it’s elephant sedatives. National Post. 2016. Available from URL: http://nationalpost.com/news/world/elephant-sedative-carfentanil-threat (accessed August 2018).
BC Gov News. Lab test confirms carfentanil is being ingested - 2017. Available from URL: https://news.gov.bc.ca/releases/2017HLTH0020-000224 (accessed August 2018).
CBC News.’It’s scary stuff’: deadly drug carfentanil now in Winnipeg. Posted September 2016. Available from URL: https://www.cbc.ca/news/canada/manitoba/carfentanil-drug-confirm-winnipeg-1.3783861 (accessed August 2018).
Ziegler C, Laville D. Toxic opioid carfentanil linked to 15 deaths. Alberta Gov. 2016. Available from URL: https://www.alberta.ca/release.cfm?xID=449592B2D1813-CB6E-055D-0800F5C807A854D8 (accessed August 2018).
Miller A. Deadly opioid carfentanil found in drugs in Ontario after overdose: police. Global News. 2017. Available from URL: https://globalnews.ca/news/3452150/deadly-opioid-carfentanil-found-in-drugs-in-whitby-ont-after-overdose-police/ (accessed August 2018).
CBC News. Police find carfentanil in drugs seized north of Toronto. The Canadian Press, April 2017. Available from URL: http://www.cbc.ca/news/canada/toronto/carfentanil-york-region-1.4058926 (accessed August 2018).
Canadian Centre on Substance Abuse. May 2017: Progress Report on the Joint Statement of Action to Address the Opioid Crisis in Canada. Available from URL: http://www.ccsa.ca/Resource%20Library/CCSA-Addressing-Opioid-Crisis-in-Canada-Summary-Report-2017-en.pdf (accessed August 2018).
Kinetz E, Butler D. Several Chinese companies willing to export carfentanil: AP investigation. Associated Press. October 2016. Available from URL: http://www.cbc.ca/news/world/carfentanil-exports-china-ap-investigation-1.3795415 (accessed August 2018).
CBC News. China to regulate carfentanil, seen as ‘substantial step’ in curbing opioid exports. Associated Press. February 2017. Available from URL: http://www.cbc.ca/news/world/china-carfentanil-controlled-substance-list-1.3985265 (accessed August 2018).
Kreeger TJ, Arnemo JRJ. Handbook of Wildlife Chemical Immobilization. International ed. Fort Collins, CO: Wildlife Pharmaceuticals; 1999 .
Lust EB, Barthold C, Malesker MA, Wichman TO. Human health hazards of veterinary medications: information for emergency departments. J Emerg Med 2011; 40: 198-207.
Canada Minstry of Justice. Controlled Drugs and Substances Act. S.C. 1996, c.19.; 2018: 1-112.
Frost JJ, Douglass KH, Mayberg HS, et al. Multicompartmental analysis of [11C]-carfentanil binding to opiate receptors in humans measured by positron emission tomography. J Cereb Blood Flow Metab 1989; 9: 398-409.
World Health Organization. Carfentanil: Critical Review Report. Agenda Item 4.8. Expert Comm Drug Depend 39th Meet. 2017; (November): 16-20. Available from URL: http://www.who.int/medicines/access/controlled-substances/ecdd_39_meeting/en/ (accessed August 2018).
Wildlife Pharmaceuticals Incorporated. Material Safety Data Sheet. Production Description - Wildnil®.
Prekupec MP, Mansky PA, Baumann MH. Misuse of novel synthetic opioids: a deadly new trend. J Addict Med 2017; 11: 256-65.
Feasel MG, Wohlfarth A, Nilles JM, Pang S, Kristovich RL, Huestis MA. Metabolism of carfentanil, an ultra-potent opioid, in human liver microsomes and human hepatocytes by high-resolution mass spectrometry. AAPS J 2016; 18: 1489-99.
Minkowski CP, Epstein D, Frost JJ, Gorelick DA. Differential response to IV carfentanil in chronic cocaine users and healthy controls. Addict Biol 2012; 17: 149-55.
Uddayasankar U, Lee C, Oleschuk C, Eschun G, Ariano RE. The pharmacokinetics and pharmacodynamics of carfentanil after recreational exposure: a case report. Pharmacotherapy 2018; 38: e41-5.
Drugbank. Carfentanil. August 2, 2017. Available from URL: www.drugbank.ca/drugs/DB01535 (accessed August 2018).
Shaw M, Carpenter J, Leith D. Complications with the use of carfentanil citrate and xylazine hydrochloride to immobilize domestic horses. J Am Vet Med Assoc 1995; 206: 833-6.
Haigh JC, Gates CC. Capture of wood bison (Bison bison athanascae) using carfentanil-based mixtures. J Wildl Dis 1995; 31: 37-42.
Miller MW, Wild MA, Lance WR. Efficacy and safety of naltrexone hydrochloride for antagonizing carfentanil citrate immobilization in captive Rocky Mountain elk (Cervus elaphus nelsoni). J Wildl Dis 1996; 32: 234-9.
Caspi J, Klausner JM, Safadi T, Amar R, Rozin RR, Merin G. Delayed respiratory depression following fentanyl anesthesia for cardiac surgery. Crit Care Med 1988; 16: 238-40.
Labroo RB, Paine MF, Thummel KE, Kharasch ED. Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for individual variablity in disposition, efficacy, and drug interactions. Drug Metab Dispos 1997; 25: 1072-80.
Riches JR, Read RW, Black RM, Cooper NJ, Timperley CM. Analysis of clothing and urine from Moscow theatre siege casualties reveals carfentanil and remifentanil use. J Anal Toxicol 2012; 36: 647-56.
Müller S, Nussbaumer S, Plitzko G, et al. Recreational use of carfentanil – a case report with laboratory confirmation. Clin Toxicol (Phila) 2017; 56: 151-2.
Van Bever WF, Niemegeers CJ, Schellekens KH, Janssen PA. N-4-substituted 1-(2-arylethyl)-4-piperidinyl-N-phenylpropanamides, a novel series of extremely potent analgesics with unusually high safety margin. Arzneimittelforschung 1976; 26: 1548-51.
Binding DB. The Binding Database - 2017. Available from URL: https://www.bindingdb.org/jsp/dbsearch/PrimarySearch_ki.jsp?energyterm=kJ/mole&tag=lidki&monomerid=50012477&column=KI&startPg=0&Increment=50&submit=Search (accessed August 2018).
Wax PM, Becker CE, Curry SC. Unexpected “gas” casualties in Moscow: a medical toxicology perspective. Ann Emerg Med 2003; 41: 700-5.
Akorn. Fentanyl Citrate Injection Product Monograph - 2012. Available from URL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/016619s034lbl.pdf (accessed August 2018).
Burns G, DeRienz RT, Baker DD, Casavant M, Spiller HA. Could chest wall rigidity be a factor in rapid death from illicit fentanyl abuse? Clin Toxicol (Phila) 2016; 54: 420-3.
Çoruh B, Tonelli MR, Park DR. Fentanyl-induced chest wall rigidity. Chest 2013; 143: 1145-6.
George AV, Lu JJ, Pisano MV, Metz J, Erickson TB. Carfentanil–an ultra potent opioid. Am J Emerg Med 2010; 28: 530-2.
Shoff EN, Zaney ME, Kahl JH, Hime GW, Boland DM. Qualitative identification of fentanyl analogs and other opioids in postmortem cases by UHPLC-ion trap-MSn. J Anal Toxicol 2017; 41: 484-92.
Mounteney J, Giraudon I, Denissov G, Griffiths P. Fentanyls: are we missing the signs? Highly potent and on the rise in Europe. Int J Drug Policy 2015; 26: 626-31.
Swanson DM, Hair LS, Strauch Rivers SR, et al. Fatalities involving carfentanil and furanyl fentanyl: two case reports. J Anal Toxicol 2017; 41: 498-502.
Wang L, Bernert JT. Analysis of 13 fentanils, including sufentanil and carfentanil, in human urine by liquid chromatography-atmospheric-pressure ionization-tandem mass spectrometry. J Anal Toxicol 2006; 30: 335-41.
Canadian Centre on Substance Abuse. CCENDU Bulletin: Novel Synthetic Opioids in Counterfeit Pharmaceuticals and Other Illicit Street Drugs; 2016. Available from URL: http://www.ccdus.ca/Resource%20Library/CCSA-CCENDU-Novel-Synthetic-Opioids-Bulletin-2016-en.pdf#search=all%28Novel%20synthetic%20opioids%20in%20counterfeit%20pharmaceuticals%29 (accessed August 2018).
Sandoz Canada Incorporated. S.O.S. Naloxone hydrochloride injection. 2017: 1-26. Available from URL: https://pdf.hres.ca/dpd_pm/00039142.PDF (accessed August 2018).
Lavonas EJ, Drennan IR, Gabrielli A, et al. Part 10: Special circumstances of resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015; 132: S501-18.
Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila) 2008; 46: 501-6.
Moss MJ, Warrick BJ, Nelson LS, et al. ACMT and AACT position statement: preventing occupational fentanyl and fentanyl analog exposure to emergency responders. Clin Toxicol (Phila) 2017; 56: 297-300.
Melichar JK, Nutt DJ, Malizia AL. Naloxone displacement at opioid receptor sites measured in vivo in the human brain. Eur J Pharmacol 2003; 459: 217-9.
Cole JB, Nelson LS. Controversies and carfentanil: we have much to learn about the present state of opioid poisoning. Am J Emerg Med 2017; 35: 1743-5.
United States Drug Enforcement Administration. Drug Enforcement Administration Report: DEA Warning to Police and Public: Fentanyl Exposure Kills. 2017. available from URL: https://www.dea.gov/press-releases/2016/06/10/dea-warning-police-and-public-fentanyl-exposure-kills (accessed August 2018).
Justice Institute of British Columbia. Fentanyl Safety - 2016. Available from URL: https://www.fentanylsafety.com/ (accessed August 2018).
Drug Enforcement Administration. U.S. Department of Justice. Drug Enforcement Administration. Furanyl-fentanyl·acryl-fentanyl·acetyl-fentanyl. A Briefing Guide for First Responders. Carfentanil-3-methylfentanyl & synthetic opioids - 2017. Available from URL: https://www.iaclea.org/assets/uploads/pdfs/Fentanyl_BriefingGuide_June2017.pdf (accessed August 2018).
Centre for Disease Control and Prevention. Fentanyl: Preventing Occupational Exposure to Emergency Responders - 2016. Available from URL: https://www.cdc.gov/niosh/topics/fentanyl/risk.html (accessed August 2018).
Conflicts of interest
None declared.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Jessica Leen conducted the literature review and drafted the article. Jessica Leen and David Juurlink critically reviewed and revised the manuscript.
Funding
None.
Author information
Authors and Affiliations
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is accompanied by an editorial. Please see Can J Anesth 2019; 66: this issue.
Rights and permissions
About this article
Cite this article
Leen, J.L.S., Juurlink, D.N. Carfentanil: a narrative review of its pharmacology and public health concerns. Can J Anesth/J Can Anesth 66, 414–421 (2019). https://doi.org/10.1007/s12630-019-01294-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-019-01294-y