Abstract
Purpose
Intravenous fluid management for deceased donor kidney transplantation is an important, modifiable risk factor for delayed graft function (DGF). The primary objective of this study was to determine if goal-directed fluid therapy using esophageal Doppler monitoring (EDM) to optimize stroke volume (SV) would alter the amount of fluid given.
Methods
This randomized, proof-of-concept trial enrolled 50 deceased donor renal transplant recipients. Data collected included patient characteristics, fluid administration, hemodynamics, and complications. The EDM was used to optimize SV in the EDM group. In the control group, fluid management followed the current standard of practice. The groups were compared for the primary outcome of total intraoperative fluid administered.
Results
There was no difference in the mean (standard deviation) volume of intraoperative fluid administered to the 24 control and 26 EDM patients [2,307 (750) mL vs 2,675 (842) mL, respectively; mean difference, 368 mL; 95% confidence interval (CI), − 87 to + 823; P = 0.11]. The incidence of complications in the control and EDM groups was similar (15/24 vs 17/26, respectively; P = 0.99), as was the incidence of delayed graft failure (8/24 vs 11/26, respectively; P = 0.36).
Conclusions
Goal-directed fluid therapy did not alter the volume of fluid administered or the incidence of complications. This proof-of-concept trial provides needed data for conducting a larger trial to determine the influence of fluid therapy on the incidence in DGF in deceased donor kidney transplantation.
Trial registration
www.clinicaltrials.gov (NCT02512731). Registered 31 July 2015.
Résumé
Objectif
La gestion des liquides intraveineux pour la transplantation de rein de donneur décédé est un facteur de risque modifiable important du retard de fonctionnement du greffon (DGF). L’objectif principal de cette étude était de déterminer si un traitement liquidien utilisant un monitorage par Doppler transœsophagien (EDM) pour optimiser le volume d’éjection cardiaque modifierait la quantité de liquides administrée.
Méthodes
Cette étude randomisée de preuve de concept a inclus 50 patients receveurs de greffe de rein provenant de donneurs décédés. Les données collectées ont inclus les caractéristiques démographiques des patients, l’administration de liquides, les données hémodynamiques et les complications. L’EDM a été utilisé pour optimiser le volume d’éjection dans le groupe EDM. Dans le groupe contrôle, la gestion des liquides a suivi les normes de pratique actuelles. Le critère d’évaluation principal qui était la quantité totale de liquide administrée en peropératoire a été comparé entre les groupes.
Résultats
Il n’y a pas eu de différence de volume moyen (écart-type) de liquide administré en cours d’intervention entre les 24 témoins et les 26 patients du groupe EDM (respectivement 2 307 [750] mL contre 2 675 [842] mL; différence des moyennes, 368 mL; intervalle de confiance [IC] à 95 % : − 87 à + 823; P = 0,11). L’incidence des complications a été similaire dans le groupe contrôle et dans le groupe EDM (respectivement, 15/24 contre 17/26; P = 0,99), tout comme l’incidence des échecs tardifs des greffes (respectivement, 8/24 contre 11/26; P = 0,36).
Conclusions
Le traitement avec gestion des liquides axé basé sur l’optimisation du volume d’éjection n’a pas modifié le volume de liquides administrés ou l’incidence des complications. Cette étude de preuve de concept procure les données nécessaires à la réalisation d’une plus grande étude visant à déterminer l’influence du traitement liquidien sur l’incidence du DGF après transplantation rénale de donneur décédé.
Enregistrement de l’essai clinique
www.clinicaltrials.gov (NCT02512731). Enregistré le 31 juillet 2015.
Similar content being viewed by others
Delayed graft function (DGF), defined as the need for dialysis within the first week after kidney transplantation, is associated with an increase in morbidity, mortality, and reduced graft survival.1,2,3 Delayed graft function occurs in 30-50% of kidney transplants from deceased donors and its incidence may be increasing as the use of extended criteria and alternate source donor organs increases (e.g., death after controlled and uncontrolled circulatory death donors).4,5,6 Importantly, donor perioperative and recipient variables have been associated with DGF4,5 and some of these putative risk factors are within the control of the anesthesiologist. To improve early graft function, it is incumbent upon us to identify and improve modifiable risk factors for DGF.
A comprehensive effort to develop an evidenced-based pathway for the management of kidney transplant recipients must include intraoperative fluid therapy. Evidence supporting the importance of fluid management in kidney transplant surgery found optimized cardiac preload7 and increasing albumin use8,9,10 improved early graft function. These initial reports found a dramatic reduction in DGF (from 34% to 9%), but it is difficult to draw reliable conclusions from these poorly risk-adjusted retrospective investigations. Despite a lack of convincing evidence, hemodynamic goals such as a central venous pressure (CVP) > 8 mmHg and a mean arterial pressure (MAP) within 20% of baseline MAP are commonly utilized to guide perioperative fluid administration for kidney transplantation.11 Nonetheless, clinical evidence in other surgical settings suggests that the CVP is unreliable as both a marker for the need for fluid administration and a proxy of fluid responsiveness.12 Similarly, the use of MAP may be flawed if the treatment of hypotension relies solely on cardiac preload, ignoring other causes of hypotension such as vasodilation or a reduction in cardiac contractility. The administration of fluid without hemodynamic improvement is likely only to increase the risk of complications associated with the excess fluid (e.g., via increasing interstitial tissue edema). The importance of optimizing perioperative fluid therapy and cardiac output is supported by the association of factors such as hypotension, reduced cardiac filling pressures,13,14 pulmonary pressure,15 and preoperative dialysis with significant weight loss with higher incidence of DGF and graft failures.16
The main hypothesis of this proof-of-concept trial was that fluid management based on hemodynamic management would change the amount of fluid administered to kidney transplant recipients. Improved fluid management may in turn improve graft function, but a much larger trial would be required to confirm this hypothesis. Therefore, a secondary objective of this trial was to understand the feasibility of conducting a larger trial in the future.
Methods
This is a single-centre, randomized, proof-of-concept trial comparing the amount of intravenous fluid administered during surgery between an intervention using esophageal Doppler monitoring (EDM group) vs controls. The trial was approved (approval number FYI 11-0055-A; 1 August 2011) by the University Health Network research ethics board (REB) and was registered on Clinicaltrials.gov (NCT02512731). No departures from the REB protocol were submitted, but the method for obtaining informed consent was modified to include a mailed letter of introduction to the study protocol to patients awaiting kidney transplantation. This modification allowed us to inform a potential study participant of the study prior to their surgical date as stipulated by the REB. Without this modification, we were unable to consent any patients prior to their date of transplant between August 2011 and July 2013. The patients assessed during this period were not included in the screened patient population considered for the study.
Patient population
We assessed the study eligibility of all adult patients awaiting kidney transplantation from a deceased donor at the Toronto General Hospital from 1 June 2013 to 23 July 2015. Patients on the transplant waiting list were contacted by mail and a general description of the study protocol included with the letter of introduction. Contact information was included in the communication and the perioperative research team was available to answer questions. Patients were asked to sign informed consent only after they indicated they had received and understood our mailing. Patients were also enrolled in the preoperative clinics, but the consent was re-obtained if the original signature was obtained more than 1 month prior to surgery.
Patients were included if they were ≥ 18 yr of age and listed for deceased-donor kidney transplantation. Exclusion criteria included prior esophageal disease (including cancer, esophageal stricture, varices or diverticulum, or prior esophageal surgery), upper airway surgery, aortic coarctation, significant valvular heart disease (i.e., limiting functional capacity), clinically significant cardiac arrhythmias (i.e., atrial fibrillation), and the need for multi-organ transplantation (e.g., kidney-pancreas).
Randomization, allocation, and blinding
Computer-generated sequences in blocks of eight were used for randomization and group allocation envelopes were prepared before the start of the patient enrolment by a research assistant. These sealed, opaque envelopes were sequentially numbered and brought to the operating room for consented patients. Envelopes were opened in the operating room after induction of anesthesia by the anesthesiologist responsible for intraoperative care, thus assigning patients to either the control or EDM-guided groups. The attending anesthesiologist was not given access to the stroke volume (SV) measurements in the control group. Surgical and nephrology teams were blinded to the patients’ assignment as were the anesthesiologists involved in postoperative care (e.g., attending in the postanesthesia care unit [PACU] or the acute pain service). Outcome data were adjudicated by the senior author (S.A.M.) who was blinded to group allocation and patient identifiers.
Data collection
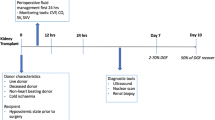
Baseline data were collected on each patient including demographics (i.e., age, gender, height, weight, and ethnicity), etiology of end-stage renal disease, duration of end-stage renal disease, duration and type of renal replacement therapy, type and date of last dialysis prior to surgery, and co-morbidities (including previous transplants). The intraoperative data included hemodynamic data and intraoperative complications. Postoperatively, data were collected on serum creatinine on postoperative day (POD) 1, 3, and 7 and/or discharge (whichever came first) and Clavien-Dindo grade ≥ II complications (i.e., respiratory—prolonged postoperative ventilation or reintubation; cardiovascular—new onset arrhythmia, myocardial infarction, pulmonary embolism, cardiogenic pulmonary edema, cardiac arrest; neurologic—delirium or stroke; infectious—surgical site infection, pneumonia, urinary tract infections; early reoperation, hemorrhage, length of hospital stay, and DGF).17 Donor (and graft) data collected included donor age, sex, creatinine level immediately prior to donation, cold and warm ischemic time, as well as preservation solution and type (static storage or hypothermic machine perfusion).
Anesthetic technique
On arrival to the operating room, routine non-invasive monitors (electrocardiography, noninvasive blood pressure, heart rate pulse oximetry [blood oxygen saturation; SpO2]), and invasive arterial blood pressure monitoring were applied.18 Anesthetic management was at the discretion of the attending anesthesiologist. Mechanical ventilation was commenced with a tidal volume of 6-8 mL·kg−1 of air/oxygen mixture. Positive end expiratory pressure was applied if clinically indicated. Respiratory rate and the fraction of inspired oxygen were set to keep end-tidal carbon dioxide in the range of 35-45 mmHg and the SpO2 > 95%. Normothermia (36-37°C nasal or oropharyngeal) was maintained with forced air warming blankets and a fluid warmer. After induction of general anesthesia, a CVP catheter was placed in an internal jugular vein.
All study patients had an EDM probe (CardioQ, Deltex, Provincial Medical Supplies Ltd, Georgetown, ON, Canada) placed after induction of anesthesia. The tip of the probe was positioned 35-40 cm from the teeth and adjusted until the highest Doppler velocity signal was recorded.
All patients received 20% mannitol (0.5-0.75 mg·kg−1) immediately prior to removal of the recipient vascular cross-clamp. No restriction of the use of inotropes was made providing fluid optimization was conducted first. The volume of intraoperative fluid included crystalloid and colloid (albumin 5%). The EDM probe was removed at the end of the intraoperative period.
Patients were extubated in the operating room or in the PACU according to the clinical judgment of the attending anesthesiologist. All patients were transferred to the PACU after surgery and received the usual standard of care. Postoperative fluid management for both groups relied on the information available for all patients undergoing renal transplantation (i.e., fluid balance and hemodynamic measurements available routinely). This management was shared between the transplant nephrologist and the attending anesthesiologist in the PACU.
In general, fluid management during kidney transplantation was left to the discretion of the attending anesthesiologist. According to the current practice, suggested hemodynamic target values were a CVP ≤ 12-15 mmHg and a systolic blood pressure > 100 mmHg. Amount, type, and timing of administered fluids were left to the discretion of the attending anesthesiologist.
Fluid management in the EDM group
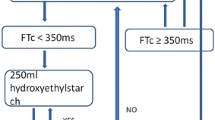
Following optimization of the EDM signal, fluid administration was managed according to an algorithm immediately after induction of anesthesia and prior to skin incision (Fig. 1). Briefly, after establishing baseline hemodynamic values, a 500-mL aliquot of crystalloid was administered over two to five minutes and this was referred to as a volume-loading step (VLS). The patient was considered a fluid responder if the SV increased by 10% or more during or after a VLS. The provider was instructed to avoid any changes in management that might otherwise affect SV (e.g., narcotic administration) during a VLS. The VLS was repeated until the increase in SV was < 10%. This was considered as the optimized SV. Additional fluid boluses were given whenever SV decreased by 25% for more than one minute from the optimized SV in order to reach the optimized SV ± 25%. There was no maximum number of VLSs permitted, but the attending anesthesiologist was asked to use clinical judgment with each step. A continuous infusion of 0.5 mL·kg−1·hr−1 of crystalloid solution was run throughout the case. The choice of crystalloid solution was at the discretion of the attending anesthesiologist.
In addition to the above fluid management strategies, patients in both groups received a focused transthoracic echocardiogram (FTE). An anesthesiologist not involved with the care of the patient, and blinded to the patient’s allocation, conducted a FTE in the preoperative holding area prior to transplantation to assess the baseline left and right ventricular function, presence of a pericardial effusion, and baseline intravascular volume status (change in inferior vena cava [IVC] diameter with respiration) and cardiac function. An FTE was repeated in the PACU and on the first or second POD. All FTE examinations were conducted by a certified focused ultrasonographer. Results of this assessment with regard to cardiac function were communicated to the attending anesthesiologist if they differed in any substantial way from the preoperative assessment.
Fluid management in the control group
The EDM was placed and the signal optimized after the central line had been placed and the patient was prepared for surgery. The EDM measurements were continuously recorded and downloaded to a laptop computer after each case, but were not available to the perioperative team. The EDM was covered with an opaque screen, which allowed the research staff to record SV values every 30 min to ensure the correct positioning of the esophageal probe. If the Doppler signal was not adequate, it was optimized by the research staff, but kept hidden from the clinical team.
Outcomes
The primary outcome was the volume of crystalloid solution administered intraoperatively as recorded from the anesthesia chart and verified by the research assistant. The secondary outcomes included: intraoperative SV; the cardiac index (CI) (CI = heart rate × SV index): the volume of fluid administered in the first 12 hr after surgery; the incidence of DGF (defined as the need for dialysis within the first week of transplantation); plasma creatinine concentration at the end of surgery and on POD 1, 3, 7, and at discharge; the incidence of intra- and postoperative complications.
In addition to the above, this study was conducted to determine if we could feasibly recruit sufficient numbers of patient to test the hypothesis that intraoperative fluid management could alter the incidence of DGF following cadaveric kidney transplantation. To this end, the rate of recruitment was recorded and reported.
Statistical analysis
Our sample size estimate was based on data collected retrospectively from charts of patients (n = 328) who underwent deceased donor kidney transplantation at Toronto General Hospital between January 2000 and June 2007 where the mean (standard deviation [SD]) intraoperative fluid administered was 2,256 (1,095) mL. Using a desired power of 80% and an alpha of 0.05, a sample size of 23 patients in each group would be needed to detect an 800 mL (36%) change in volume of fluid administered intraoperatively between the EDM intervention and control groups. Two patients were added to each group to account for possible patient drop out. No interim analysis was planned and no anticipated stopping guidelines were implemented.
Continuous variables are presented as mean (SD) and median [interquartile range (IQR)]. Categorical variables are reported as percentage. Normal distribution was assessed using a skewness/kurtosis test for normality if the sample was greater than six units; if not, a Shapiro-Wilk test was used. Comparison between categorical variables was performed by the Fisher’s exact test. For continuous variables, including the primary endpoint, a Student t-test was used for the normally distributed variables, and a Wilcoxon rank sum test was used for non-parametric variables.
An analysis of variance for repeated measures was used to compare the creatinine values after surgery. We considered the group as the between-subject effect and the subject nested in the group its error term. The within-subject factor was time with its error term being the residual error for the model.
Differences in CI over time between EDM and the control group were evaluated by a repeated measures mixed model because of the differences in length of surgery and therefore differences in the number of observations of CI over time were clustered at patient level. The level of significance was set at P < 0.05. Statistical analysis was done with the Intercooled Stata 12.0 statistical package (StataCorp, College Station, TX, USA).
Results
On 10 April 2013 letters of introduction were sent out to patients on the kidney transplant wait list. Three hundred thirty-two (55%) patients responded to the letter and were thereby considered screened for the trial (Fig. 2). Of the 332 patients screened, 172 (52%) agreed to participate and 160 (48%) refused. Of the 172 patients that agreed to participate in the study, 50 (29%) were randomized and the remainder did not come to transplant during the recruitment period. The first patient consented and randomization into the study took place on 15 July 2013. One patient from the interventional group did not receive the EDM, but was analyzed as per intention to treat.
Of the patients recruited and randomized, there were no observable differences between the two groups in their preoperative variables (Table 1). As for the primary endpoint, there was no significant difference in the mean (SD) volume of crystalloid administered in the operating room between the control and EDM groups [2,675 (841) mL vs 2,307 (750) mL, respectively; mean difference, 368 mL; 95% confidence interval, − 87 to + 823; P = 0.11] (Table 2). No deviations from the fluid algorithm were reported by the clinicians. Nonetheless, while there was no statistical separation between the two groups, the volume of fluid administered in the EDM group was numerically lower (Fig. 3). This was true no matter how we expressed the volume of fluid administered—i.e., in mL or in mL·kg−1 (which normalize the data for differences in patient weight).
There was no difference in the surgical variables (i.e., warm and cold ischemia time and length of surgery) (Table 3). Despite the similar amount of fluid given to the two groups, the CI in the EDM group was consistently higher than the CI in the control group (Fig. 4). Nonetheless, when considered as a whole, and regardless of differences in length of surgery, we did not find a statistically significant separation of the mean CI between the two groups at any time point (coefficient of variability, 1.95; 95% confidence interval, -6.41 to 6.17; P = 0.65).
The hospital length of stay and number of complications were the same in both groups (Table 3). The one exception was the number of the cardiovascular events, which were more frequent in the EDM group (Table 3). There appeared to be a more rapid decrease in the creatinine after transplantation in the EDM group (Fig. 5). Nonetheless, the reduction in serum creatinine was not statistically different in the EDM group at any time point after transplantation, [f(1,48) = 0.75; P = 0.39] or treatment by time [f (5,5) = 0.69, P = 0.63].
Complete FTE studies were available on 31, 33, and 22 subjects preoperatively, immediately postoperatively, and POD 1 or 2, respectively (Table 4). There were no significant differences between the two groups and the incidence of fluid responsiveness as determined by > 50% variability in the IVC diameter (Table 4). Notably, four of 19 patients in the EDM group had significantly impaired left (n = 4) and/or right ventricular function (n = 1) while cardiac function was within normal limits in all patients in the control group (Table 4).
Discussion
The study was powered to determine if the use of the EDM to guide fluid therapy would change therapy or the amount of fluid administered in the operating room by 36% and this hypothesis was rejected. There are many explanations for this, but the central one may be the fluid algorithm itself. Following fluid optimization, our algorithm allowed the SV to fluctuate to a value 25% lower than the optimized SV before fluid administration was considered. In contrast, the majority of goal-directed fluid algorithms maintain the SV within 10% of the optimized value, which has been found to increase the amount of fluid administered and in some cases has led to fluid overload and poor recovery.19,20,21 A 25% reduction in SV is equivalent to the change in SV seen when a subject changes position from a recumbent to a 30º head-up position.22 Setting the indication for fluid therapy at a more forgiving cut-off or goal after fluid optimization should minimize the risk of fluid overload. It should also be mentioned that the coefficient of variability in the measurement of SV by the EDM is > 10% and therefore using a criterion for transfusing additional fluid of a 25% change in the SV adds an additional safety measure by staying within the limits of detection of the EDM.23 A patient population with end-stage renal failure might be more susceptible to the fluid overload and therefore a conservation fluid therapy is appropriate.
While we found no significant separation of the mean volume of fluid administered in the two groups, there appeared be differences in the distribution of the fluid volume administered as depicted in Fig. 3. The control group had a greater range of fluid volumes than the EDM group. Nonetheless, our study was not powered to compare groups with this methodology, and this potential treatment effect must be interpreted cautiously and requires further confirmation in a larger trial.
Other reasons for not finding an increase in fluid administration in the EDM group include a potential Hawthorne effect, a process where investigators change practice based on exposure to the treatment protocol. In our case, clinicians may have learned that more fluid is a safe approach even in the control group. Finally, the targeted change in fluid administration of 36% or approximately 800 mL based on a historic kidney transplant cohort from our center used in the original sample size calculation may have been unrealistic.
It should be clearly stated that we were not able to show a difference in the amount of fluid administered to our patient population. Nonetheless, the importance of goal-directed fluid therapy should not be dismissed as its beneficial effects have been seen in other similar trials. In the systematic meta-analysis by Corcoran et al., patients undergoing a major abdominal surgical procedure had improved outcomes—i.e., shorter length of hospital stay, time to recovery of bowel function, and fewer infectious complications24—if they were managed with a goal-directed strategy rather than a “liberal” standard fluid strategy. Similar amounts of fluid were administered to both groups and therefore the benefit of giving fluid to only fluid-responsive patients may be more important than the volume of fluid administered. In may be that at our institution the control group is managed more or less liberally than at other centres and therefore a multi-centered trial is required to find the benefits goal directed fluid therapy in the renal transplant patient or even differences in fluid therapy itself.
Our study was underpowered to look at secondary outcomes such as creatinine elimination or hemodynamic changes and no differences were found between the groups (Figs. 4 and 5). These measurements should be reconsidered in a future trial. Lower creatinine in the immediate postoperative period has been considered a better predictor of long-term graft function than the need for dialysis in the first week after transplantation.25,26 The impact of reducing DGF and improving graft function has an impact that persists for many years beyond post-transplantation.2,3 When the definition of DGF is considered as a < 10% reduction in serum creatinine for the first three days after transplantation, we found a significant difference between the groups (25 vs 15%; P = 0.049, control vs EDM group, respectively). This post hoc analysis is only marginally significant and should be considered cautiously. Nonetheless, this finding serves to underscore the possible benefit of goal-directed fluid therapy in renal transplantation even if management has not changed significantly as measured by total fluid administered in the operating room.
This proof-of-concept trial was not powered to look at differences in DGF or complications, but the increase in cardiovascular complications seen in the EDM group does raise concern. Even though the statistical difference may be an error due to multiple testing (i.e., a type II error), we would be hesitant to change the fluid algorithm to more aggressively optimize the SV. Clearly this will be an important concern for a larger trial. In addition, the definition of cardiovascular event was very broad for this proof-of-concept trial and the cardiovascular events found in this study included pulmonary embolism, atrial dysthymias, cardiac arrest, and any rise in troponin when troponin was measured. It will be important to specify the cardiac event (e.g., myocardial infarction) and routinely measure serial troponin in a larger trial.
A final limitation to this study is the familiarity of clinicians with EDM. Unlike some practice settings, EDM is not routinely used in our hospital and therefore clinicians may not have been able to use it effectively or at least understand its strengths and weaknesses. We instructed clinicians to use their clinical judgement to override the device and the fluid algorithm, but no overrides were reported. Therefore, either the fluid algorithm was mimicking local practice or clinicians were not reporting overrides of the protocol. In either case, fluid therapy in both groups would be similar. Nonetheless, the study coordinator was available for each case and should have observed protocol deviations if they occurred so we might conclude that the EDM group and control group (i.e., local clinical practice) had similar goals for fluid therapy.
The systematic use of FTE assessment in the perioperative period for renal transplant patients has the potential to add a great deal of information for fluid management and patient care perioperatively. We did not find any significant difference between the groups, and a basic FTE does not appear to be sufficiently sensitive to be included into routine clinical care for kidney transplant recipients (Table 4). Nonetheless, the observed impaired cardiac function in four patients in the EDM group suggests that FTE may help guide patient management in the postoperative period.
To show the effectiveness of goal-directed therapy to reduce the incidence or DGF by 25% (i.e., an absolute risk reduction from 33% to 25%), a trial would require more than 500 patients in each group; clearly a multi-centred trial would be required. At our centre, we conduct nearly 200 deceased donor kidney transplants per year and of those we would anticipate enrolling a minimum 50 patients per year. To optimize patient recruitment, we would need to focus on patient awareness and attention. By including another 10-12 similar-sized centres, it would be reasonable to assume the completion of a sufficiently powered study to test the hypothesis that goal-directed fluid management might improve a major outcome in kidney transplantation in two years.
Future studies in renal transplantation should also extend into the postoperative period. The use of the EDM would need to be reconsidered unless it can be appropriately paired with a cardiac output monitor that can remain with the patient for the early recovery period. This would also require the support of the attending nephrologist, but there is evidence that protocol-driven fluid therapy is better than protocol-based therapy for postoperative outcomes.27
Conclusions
Nearly one third of patients experience DGF following deceased donor renal transplantation and this can have a major impact on graft and patient survival.3 We have developed an effective strategy to recruit deceased-donor kidney transplant recipients to clinical trials examining patient care and in particular fluid therapy. Enhanced recovery programs are believed to improve patient recovery through the application of best evidence to perioperative care, the standardization of management, and improved communication among services (i.e., medicine, surgery, and anesthesia).14 The same should be true for kidney transplantation, and by conducting a trial of goal-directed fluid therapy in this population, a central component of any enhanced recovery program, in a large randomized-controlled trial, we will be able to determine the benefits of fluid therapy and allow for the standardization of perioperative care. This proof-of-concept trial has given us the data needed to develop such a trial and the benefactor will be our kidney transplant population and healthcare system.
References
Yarlagadda SG, Coca SG, Garg AX, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant 2008; 23: 2995-3003.
Tapiawala SN, Tinckam KJ, Cardella CJ, et al. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol 2010; 21: 153-61.
Yarlagadda SG, Coca SG, Formica RN Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant 2009; 24: 1039-47.
Keitel E, Michelon T, dos Santos AF, et al. Renal transplants using expanded cadaver donor criteria. Ann Transplant 2004; 9: 23-4.
Heilman RL, Mathur A, Smith ML, Kaplan B, Reddy KS. Increasing the use of kidneys from unconventional and high-risk deceased donors. Am J Transplant 2016; 16: 3086-92.
Demiselle J, Augusto JF, Videcoq M, et al. Transplantation of kidneys from uncontrolled donation after circulatory determination of death: comparison with brain death donors with or without extended criteria and impact of normothermic regional perfusion. Transpl Int 2016; 29: 432-42.
Carlier M, Squifflet JP, Pirson Y, Decocq L, Gribomont B, Alexandre GP. Confirmation of the crucial role of the recipient’s maximal hydration on early diuresis of the human cadaver renal allograft. Transplantation 1983; 36: 455-6.
Abdallah E, El-Shishtawy S, Mosbah O, Zeidan M. Comparison between the effects of intraoperative human albumin and normal saline on early graft function in renal transplantation. Int Urol Nephrol 2014; 46: 2221-6.
Dawidson IJ, Sandor ZF, Coorpender L, et al. Intraoperative albumin administration affects the outcome of cadaver renal transplantation. Transplantation 1992; 53: 774-82.
Willms CD, Dawidson IJ, Dickerman R, Drake D, Sandor ZF, Trevino G. Intraoperative blood volume expansion induces primary function after renal transplantation: a study of 96 paired cadaver kidneys. Transplant Proc 1991; 23(1 Pt 2): 1338-9.
Schmid S, Jungwirth B. Anaesthesia for renal transplant surgery: an update. Eur J Anaesthesiol 2012; 29: 552-8.
Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 2008; 134: 172-8.
Snoeijs MG, Wiermans B, Christiaans MH, et al. Recipient hemodynamics during non-heart-beating donor kidney transplantation are major predictors of primary nonfunction. Am J Transplant 2007; 7: 1158-66.
Thomsen HS, Lokkegaard H, Munck O. Influence of normal central venous pressure on onset of function in renal allografts. Scand J Urol Nephrol 1987; 21: 143-5.
Luciani J, Frantz P, Thibault P, et al. Early anuria prevention in human kidney transplantation. Advantage of fluid load under pulmonary arterial pressure monitoring during surgical period. Transplantation 1979; 28: 308-12.
Van Loo AA, Vanholder RC, Bernaert PR, Vermassen FE, Van der Vennet M, Lameire NH. Pretransplantation hemodialysis strategy influences early renal graft function. J Am Soc Nephrol 1998; 9: 473-81.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205-13.
Dobson G, Chong M, Chow L, et al. Guidelines to the practice of anesthesia - revised edition 2017. Can J Anesth 2017; 64: 65-91.
Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg 2011; 112: 1392-402.
Challand C, Struthers R, Sneyd JR, et al. Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br J Anaesth 2012; 108: 53-62.
Lai CW, Starkie T, Creanor S, et al. Randomized controlled trial of stroke volume optimization during elective major abdominal surgery in patients stratified by aerobic fitness. Br J Anaesth 2015; 115: 578-89.
Jans O, Tollund C, Bundgaard-Nielsen M, Selmer C, Warberg J, Secher NH. Goal-directed fluid therapy: stroke volume optimisation and cardiac dimensions in supine healthy humans. Acta Anaesthesiol Scand 2008; 52: 536-40.
Schober P, Loer SA, Schwarte LA. Perioperative hemodynamic monitoring with transesophageal Doppler technology. Anesth Analg 2009; 109: 340-53.
Corcoran T, Rhodes JE, Clarke S, Myles PS, Ho KM. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg 2012; 114: 640-51.
Akkina SK, Connaire JJ, Israni AK, Snyder JJ, Matas AJ, Kasiske BL. Similar outcomes with different rates of delayed graft function may reflect center practice, not center performance. Am J Transplant 2009; 9: 1460-6.
Shaw AD. Cardiac surgery-associated acute kidney injury: tools for enriching clinical trial populations. Can J Anesth 2017; 64: 793-6.
Pearse RM, Harrison DA, MacDonald N, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 2014; 311: 2181-90.
Acknowledgements
Drs. Hilary Felice and Rohan Kothari initiated the study and developed a recruitment strategy consistent with the recommendations of our Research Ethics Board and principles of informed patient consent.
Davide Corbella and Patrick Jason Toppin were supported by a departmental funding as part of the Abdominal Organ Transplantation Fellowship in Anesthesia in the Department of Anesthesia and Pain Management, Toronto General Hospital, University Health Network.
This trial was supported in part by the Dr. Earl Wynands/Fresenius Kabi Research Award from the Canadian Anesthesia Research Foundation. ClinicalTrial.gov number: NCT02512731.
Conflicts of interest
None declared.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Davide Corbella was responsible for analyzing the data and drafting the first version of the article submitted and the subsequent version. Patrick Jason Toppin developed the protocol. Nour Ayach managed the trial and obtained REB approval. Anand Ghanekar, Jeffery Schiff, Adrian Van Rensburg, and Stuart A. McCluskey developed the protocol and supported the study in their own clinical specialty. All authors participated in the revision and content editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corbella, D., Toppin, P.J., Ghanekar, A. et al. Cardiac output-based fluid optimization for kidney transplant recipients: a proof-of-concept trial. Can J Anesth/J Can Anesth 65, 873–883 (2018). https://doi.org/10.1007/s12630-018-1118-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-018-1118-y