Abstract
Purpose
Nasotracheal intubation is a widely performed technique to facilitate anesthesia induction during oral, dental, and maxillofacial surgeries. The technique poses several risks not encountered with oropharyngeal intubation, most commonly epistaxis due to nasal mucosal abrasion. The purpose of this study was to test whether the use of the Parker Flex-Tip™ (PFT) nasal endotracheal tube (ETT) with a posterior facing bevel reduces epistaxis when compared with the standard nasal RAE ETT with a leftward facing bevel.
Methods
Sixty American Society of Anesthesiologists physical status I and II patients undergoing oral or maxillofacial surgery with nasotracheal intubation were recruited. Patients were randomized to either a standard nasal RAE ETT or a PFT nasal ETT. The ETT was thermosoftened and lubricated for both study groups prior to insertion, and the size of the tube was chosen at the discretion of the attending anesthesiologist. The primary outcome was the incidence of epistaxis, with a secondary outcome of epistaxis severity (scored as none, mild, moderate, or severe). An investigator measured both outcomes five minutes after intubation was completed.
Results
Mild or moderate epistaxis was experienced by 22 of 30 (73%) patients in the PFT group compared with 21 of 30 (70%) patients in the standard nasal RAE ETT group (absolute risk reduction, 3%; 95% confidence interval, −19 to 25; P = 0.78). There were no occurrences of severe epistaxis in either group.
Conclusion
There was no difference in the incidence or severity of epistaxis following nasal intubation using the Parker Flex-Tip nasal ETT when compared with a standard nasal RAE ETT. This trial was registered at ClinicalTrials.gov, identifier: NCT02315677.
Résumé
Objectif
L’intubation nasotrachéale est une technique répandue pour faciliter l’induction de l’anesthésie pendant les chirurgies orales, dentaires et maxillo-faciales. La technique comporte plusieurs risques que l’on ne rencontre pas lors d’une intubation oropharyngée, le plus fréquent étant l’épistaxis provoquée par l’abrasion de la muqueuse nasale. L’objectif de cette étude était d’évaluer si l’utilisation d’une sonde endotrachéale nasale (SET) Parker Flex-Tip™ (PFT) munie d’un biseau à ouverture postérieure réduisait l’épistaxis par rapport à une SET RAE standard, équipée d’un biseau à ouverture vers la gauche.
Méthode
Soixante patients de statut physique ASA (American Society of Anesthesiologists) I et II subissant une chirurgie orale ou maxillo-faciale avec intubation nasotrachéale ont été recrutés. Les patients ont été randomisés aux groupes SET RAE nasale standard ou au groupe SET PFT nasale. La SET a été réchauffée pour la ramollir et lubrifiée dans les deux groupes avant l’insertion, et la taille de la sonde a été choisie à la discrétion de l’anesthésiologiste. Le critère d’évaluation principal était l’incidence d’épistaxis, et le critère d’évaluation secondaire était la gravité de l’épistaxis (notée en tant que nulle, légère, modérée ou grave). Un chercheur a mesuré les deux critères cinq minutes après la complétion de l’intubation.
Résultats
Une épistaxis légère ou modérée a été éprouvée par 22 des 30 (73 %) patients dans le groupe PFT par rapport à 21 des 30 (70 %) patients du groupe SET RAE nasale standard (réduction du risque absolu, 3 %; intervalle de confiance 95 %, −19 à 25; P = 0,78). Aucune épistaxis grave n’est survenue dans l’un ou l’autre groupe.
Conclusion
Aucune différence n’a été observée dans l’incidence ou la gravité de l’épistaxis après une intubation nasale à l’aide d’une SET nasale Parker Flex-Tip par rapport à une SET RAE nasale standard. Cette étude a été enregistrée au ClinicalTrials.gov, identifiant: NCT02315677.
Similar content being viewed by others
Nasotracheal intubation is a widely performed technique that facilitates anesthetic administration during oral, dental, and other maxillofacial surgeries. Nevertheless, nasotracheal intubation poses several challenges not encountered in standard oropharyngeal intubation, including advancing the nasal endotracheal tube (ETT) blindly through highly vascularized and delicate mucosa of the nasal passage, past protruding turbinates, and maneuvering through the sharply angulated posterior nasopharynx to access the trachea.1,2 As a result, epistaxis is a common complication of nasotracheal intubation, occurring in up to 77% of cases and ranging from only blood-tinged mucus to massive bleeding.1,2 This risk increases with the use of excessive force, larger ETTs, repeated unsuccessful attempts at intubation, and abnormalities in pharyngeal anatomy.1
Common techniques used to reduce the risks associated with nasal intubation include the use of vasoconstrictors in the nasal canal, lubrication of the ETT, selection of a smaller ETT, mechanical dilation of the nasal cavity, and deliberate maneuvering of the nasal ETT (e.g., gentle cephalad distraction of the ETT with some anticlockwise rotation to present the bevel of the ETT against the pharyngeal wall).1 Other more complicated techniques have included thermosoftening and first passing a curved-tip suction catheter through the nasopharnyx to serve as a guide for the ETT.3-6 Nevertheless, many of these techniques are complicated, time consuming, or ineffective in reducing epistaxis.
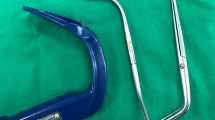
Recent studies suggest that the incidence of epistaxis can be significantly lowered with the use of a Parker Flex-Tip™ (PFT) nasal ETT (Parker Medical; Bridgewater, CT, USA), which has a posterior flexible distal tip (Fig. 1).7,8 Sanuki et al. reported a decrease in the rate of epistaxis from 35.3% to 11.8% (23.5% absolute risk reduction) with the PFT nasal ETT. Sugiyama et al. reported a decrease from 50% to 24% (26% absolute risk reduction) and as low as 4% (46% absolute risk reduction) with the addition of an anteriorly flexed stylet.7,8 It is thought that the posteriorly oriented bevel of the PFT allows the ETT to slide along the posterior wall, reducing epithelial stripping of the turbinates and septum that can occur with a standard leftward facing bevelled ETT. The soft flexible tip likely flexes on contact with the mucosa, which creates a larger surface area and thereby reduces pressure on the tissues. Furthermore, the addition of an anteriorly curved stylet may further reduce impact on the posterior pharynx as the ETT is advanced.7-9
Several methodological differences between previously published studies and our own institutional practices led us to investigate whether the potential epistaxis-reducing effects of the PFT would also be seen in our institutional practice. For example, we typically thermosoften the ETTs prior to intubation, and we do not use stylets during nasal intubation as Sugiyama et al. previously described. In addition, we have a very heterogeneous multiethnic population, which makes it inappropriate to use a size 7.5 ETT for all males and a size 7.0 ETT for all females, as described by Sugiyama, or a size 7.0 ETT for all patients, as described by Sanuki.7,8 In addition, prior studies may have used too short a time period for the epistaxis evaluation. Indeed, Xue et al. allowed at least five minutes to elapse before assessing epistaxis to allow transit time for trauma-associated blood to travel to the oropharynx.9
Accordingly, the purpose of this study was to test the hypothesis that a PFT nasal ETT with a posterior facing bevel causes less epistaxis (measured five minutes after intubation) when compared with a standard left-facing bevelled nasal RAE ETT.
Methods
After receiving ethics approval from the University of British Columbia Clinical Research Ethics Board (March, 2016), we conducted a randomized-controlled single-blinded trial. Inclusion criteria included patients with American Society of Anesthesiologists (ASA) physical status I or II, 16 yr of age and older, and undergoing maxillofacial surgery where nasal intubation was required. Exclusion criteria included previous nasal surgery or trauma, recurrent epistaxis, blood dyscrasias, or ongoing anticoagulant therapy.
After informed written consent, 60 patients were randomized to receive either a PFT nasal ETT (n = 30) or a standard Mallinckrodt™ RAE (Ring, Adair, Elwyn) nasal ETT (n = 30). Manual randomization using a sealed envelope technique was conducted in blocks of 10, with each block containing five allocations to each group. Labels indicating group designation were placed in unmarked envelopes. These envelopes were later marked with a patient number. Just prior to induction of anesthesia, the envelope was opened to reveal group allocation.
Prior to randomization, the intubating anesthesiologist (all with > five years of consultant-level experience) determined the ETT size based on a preoperative clinical assessment of the patient’s airway. Only ETT sizes 6.0, 6.5, or 7.0 were used. All ETTs were first thermosoftened in 40°C normal saline for five minutes, and a small amount of aqueous gel was applied for lubrication. The nostril that the patient reported as easier to breathe through was chosen for intubation. No topical vasoconstrictor was used. Intubation was done by direct laryngoscopy with the use of Magill forceps to guide the ETT through the cords when necessary. The proximal end of the ETT and pilot balloon were covered and the distal tip of the tube was obscured by its presence in the trachea. Five minutes after intubation, an investigator blinded to group assignment entered the operating room and scored the degree of epistaxis by direct laryngoscopy. Epistaxis was recorded using the same criteria as Sugiyama et al.—i.e., no epistaxis (no blood observed on either the surface of the tube or the posterior pharyngeal wall); mild epistaxis (blood apparent on the surface of the tube or posterior pharyngeal wall); moderate epistaxis (pooling of blood on the posterior pharyngeal wall); and severe epistaxis (a large amount of blood in the pharynx impeding nasotracheal intubation—before the epistaxis assessor entered the operating room—and necessitating urgent orotracheal intubation.7 The same investigator scored all 60 cases for the primary outcome. A modified protocol was adopted due to logistical problems in obtaining the same investigator to assess epistaxis at both time zero and five minutes—i.e., the assessments at time zero were omitted on the assumption that the assessments at five minutes would be a better indicator of the true extent of epistaxis.
Statistical analysis
Based on the results of the study by Sugiyama et al., we predicted that the incidence of epistaxis would be 50% in the standard RAE nasal ETT group and 24% in the PFT nasal ETT group.7 Allowing for an α = 0.05 (two sided) and a power of 80%, we estimated that a sample size of 27 patients per group would be required.
Baseline characteristics of the two study arms are presented as mean (standard deviation [SD]), while the incidence and severity of epistaxis are presented as percentages. The data were tested for normality using the Shapiro-Wilk test. The primary outcome, incidence of epistaxis, was tested using the Pearson’s Chi square test, with absolute risk reduction and confidence intervals (CI) calculated where applicable. The Freeman-Halton extension of the Fisher’s exact test, for a 2 × 3 contingency table, was used for testing the severity of epistaxis between groups. A P < 0.05 (two sided) was considered significant. All analyses were performed using SPSS® version 24 software (IBM, Chicago, IL, USA) and/or vassarstats.net online calculators.
Results
Sixty of the 62 patients screened (Fig. 2) from July to December 2015 consented to participate in the study. All participants completed the study and their data were analyzed for the primary outcome according to their original group allocation, with no missing data.
The groups appeared comparable regarding baseline characteristics (i.e., age, height, weight, sex, and ASA) as well as the size of the nasal ETT or the side of the nostril intubated (Table 1). Recruited patients presented for various maxillofacial surgeries (Table 2). Two patients, one patient in each group, were receiving acetylsalicylic acid (81 mg daily) at the time of their surgery, and both patients experienced moderate epistaxis.
Mild or moderate epistaxis was experienced by 22 of 30 (73%) patients in the PFT group compared with 21 of 30 (70%) patients in the standard nasal RAE ETT group (absolute risk reduction, 3%; 95% CI, −19 to 25; P = 0.78) (Table 3). The severity of epistaxis was mild in 37% (n = 11) of the PFT group compared with 40% (n = 12) in the standard nasal RAE ETT group. The severity of epistaxis was moderate in 37% (n = 11) of the PFT group compared with 30% (n = 9) in the standard nasal RAE ETT group (P = 0.90). There were no occurrences of severe epistaxis or traumatic events (e.g., turbinectomy or retropharyngeal laceration) in either group. Throughout the study, the attending anesthesiologist made no note of difficulty passing the bevel through the cords in either the PFT or the standard nasal RAE ETT group.
Discussion
Epistaxis is a common and sometimes serious risk associated with nasotracheal intubation. In this study, we found that the use of a Parker Flex-Tip nasal ETT did not provide a significant reduction in the incidence or severity of epistaxis when compared with a conventional nasal RAE ETT. These findings contrast with previous studies by Sanuki et al. and Sugiyama et al. who found that the absolute risk of epistaxis was reduced by 24% and 26%, respectively, with the PFT vs a standard nasal RAE ETT.7,8 Thermosoftening the ETT was part of our protocol, as previous studies have found that it leads to a reduction in epistaxis, and moreover, it is the usual practice in our institution.6,10 Since thermosoftening was absent in the Sugiyama and Sanuki studies,7,8 it raises the question whether this practice negated any of the previously found superiority of the PFT. This might warrant further investigation. It is also possible that our results are subject to the Hawthorne effect, i.e., due to their awareness of the study, the attending anesthesiologists may have altered their behaviour by performing the intubations more cautiously. Nevertheless, this would likely affect both groups equally, changing the overall epistaxis rates but still allowing for differences between them to remain evident. Furthermore, it is possible that the intubating anesthesiologist was biased towards either the standard ETT or the more novel PFT ETT, as they were not blinded to tube selection during intubation.
Importantly, the overall epistaxis rate in our control group (70%) was higher than reported in previous studies (35.3-50%).7,8 This difference could be due to different operator practice or increased heterogeneity of our patient characteristics given our multiethnic population (both comparative studies were based in Japan). Nevertheless, if these factors were influencing epistaxis rates, one would still expect to discern a difference between the two ETTs if the PFT was superior to the standard ETT. Another possible explanation could be that Sanuki potentially underestimated the epistaxis rates. Sanuki examined the oropharynx immediately after intubation, whereas we allowed a five-minute transit time for blood to reach the oropharynx.8 While Sugiyama did report measuring epistaxis at one and five minutes, only overall rates of bleeding were reported with no comparison between the two time points.7 Another explanation could be the size of ETT used in the Sugiyama (7.0 and 7.5) and Sanuki (7.0) studies, which were larger than the ETTs used (6.0-7.0) in our study.7,8 Although a smaller ETT size may potentially cause less trauma, the larger diameter may better tamponade any bleeding of the mucosal tissue. This warrants further investigation.
Importantly, as the PFT is considerably more expensive (approx. $14.15) than the standard nasal RAE ETT (approx. $5.35), the lack of specific clinical benefit (i.e., reduced epistaxis rate) would make the additional cost difficult to justify.
There are several limitations to our study that should be considered. First, in order to blind the independent investigator to ETT type, the proximal end and balloon port were covered and the distal tip was obscured past the cords. Nevertheless, the central portion of the ETT was visible during laryngoscopy and very minor differences in tube appearance may have been distinguishable. Unfortunately, this was unavoidable as it was not possible to assess for epistaxis adequately with the entirety of the ETT obscured. Guidelines to assess bleeding rates were very clearly defined in order to make the epistaxis assessment as objective as possible. Second, as mentioned above, epistaxis was assessed five minutes post intubation but not after extubation or at any time point postoperatively. It is possible that bleeding may have occurred after removal of the ETT due to removal of a tamponade effect or trauma during extubation. Nevertheless, after extubation, it would be difficult to differentiate surgical bleeding, particularly given the variable maxillofacial surgeries of our patient population. Furthermore, it would be challenging clinically to obtain an adequate view of the posterior pharynx with direct laryngoscopy once the patient underwent tracheal extubation and emerged from anesthesia. Lastly, epistaxis rates were not recorded based on the allocation of the various attending anesthesiologists to the different study groups, which may present a variable that was not accounted for our in our data.
In conclusion, we found that the PFT nasal ETT did not result in a significant reduction in epistaxis during nasal intubation when compared with a standard nasal RAE ETT in our patient population. Further studies are recommended to determine how thermosoftening may affect previously reported reductions in epistaxis rates and to assess the effect of ETT size on epistaxis rates following extubation.
References
Hall CE, Shutt LE. Nasotracheal intubation for head and neck surgery. Anaesthesia 2003; 58: 249-56.
Elwood T, Stillions D, Woo DW, Bradford HM, Ramamoorthy C. Nasotracheal intubation: a randomized trial of two methods. Anesthesiology 2002; 96: 51-3.
Seo KS, Kim JH, Yang SM, Kim HJ, Bahk JH, Yum KW. A new technique to reduce epistaxis and enhance navigability during nasotracheal intubation. Anesth Analg 2007; 105: 1420-4.
Watt S, Pickhardt D, Lerman J, Armstrong J, Creighton PR, Feldman L. Telescoping tracheal tubes into catheters minimizes epistaxis during nasotracheal intubation in children. Anesthesiology 2007; 106: 238-42.
Morimoto Y, Sugimura M, Hirose Y, Taki K, Niwa H. Nasotracheal intubation under curve-tipped suction catheter guidance reduces epistaxis. Can J Anesth 2006; 53: 295-8.
Kim YC, Lee SH, Noh GJ, et al. Thermosoftening treatment of the nasotracheal tube before intubation can reduce epistaxis and nasal damage. Anesth Analg 2000; 91: 698-701.
Sugiyama K, Manabe Y, Kohjitani A. A styletted tracheal tube with a posterior-facing bevel reduces epistaxis during nasal intubation: a randomized trial. Can J Anesth 2014; 61: 417-22.
Sanuki T, Hirokane M, Matsuda Y, Sugioka S, Kotani J. The Parker Flex-Tip™ tube for nasotracheal intubation: the influence on nasal mucosal trauma. Anaesthesia 2010; 65: 8-11.
Xue FS, Xion J, Yuan YJ, Wang Q. The Parker Flex-TipTM tube for nasotracheal intubation. Anesthesia 2010; 65: 417.
Lu PP, Liu HP, Shyr MH, et al. Softened endotracheal tube reduces the incidence and severity of epistaxis following nasotracheal intubation. Acta Anaesthesiol Sin 1998; 36: 193-7.
Competing interests
This work was supported by Internal Departmental Funds. The authors have no conflicts of interest to declare.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Rosie Earle and Enda Shanahan were involved in the literature search. Rosie Earle, Enda Shanahan, Himat Vaghadia, Andrew Sawka, and Raymond Tang were involved in data collection. Rosie Earle, Enda Shanahan, Andrew Sawka, and Raymond Tang were involved in manuscript preparation. Rosie Earle handled the ethics proposal. Himat Vaghadia was involved manuscript editing and study design.
Funding
Internal departmental funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Earle, R., Shanahan, E., Vaghadia, H. et al. Epistaxis during nasotracheal intubation: a randomized trial of the Parker Flex-Tip™ nasal endotracheal tube with a posterior facing bevel versus a standard nasal RAE endotracheal tube. Can J Anesth/J Can Anesth 64, 370–375 (2017). https://doi.org/10.1007/s12630-017-0813-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-017-0813-4