Abstract
Purpose
To present a case of mycobacterium infection transmitted through a heater-cooler unit during cardiac bypass surgery.
Clinical features
A 63-yr-old woman with a past medical history of aortic coarctation repair in 1963 and a mechanical aortic valve replacement in 2010 was prescribed antibiotics and steroids at an outpatient care facility in September 2015 for symptoms of an upper respiratory tract infection. Four months later, she developed malaise and intermittent fever with anemia and acute kidney dysfunction. Extensive evaluation revealed negative blood cultures but was suggestive of amyloidosis. The patient was therefore started on systemic steroids prior to being referred to us.At our institution, transesophageal echocardiography and cardiac magnetic resonance imaging revealed a normal mechanical aortic prosthesis with an aortic root abscess. The patient was started on empiric antibiotics for endocarditis. Renal biopsy revealed interstitial nephritis with one granuloma for which she was again started on high-dose steroids. The patient continued to deteriorate, with worsening renal function and pancytopenia that required daily red blood cell and platelet transfusions.Three weeks into this hospitalization, her blood cultures were reported to be positive for Mycobacterium chimera, and she was started on a four-drug regimen of rifampin, rifabutin, ethambutol, and clarithromycin, with dramatic clinical improvement.
Conclusion
Heater-cooler units manufactured by LivaNova prior to September 2014 and used during cardiopulmonary bypass have been linked to M. chimera, which causes a latent infection that may be activated and become disseminated in cases of immunosuppression related to steroid use.
Résumé
Objectif
Présenter un cas de mycobacterium transmis par un générateur thermique pendant une chirurgie de pontage coronarien.
Éléments cliniques
Une femme de 63 ans ayant subi une réparation d’une coarctation de l’aorte en 1963 et un remplacement valvulaire aortique mécanique en 2010 a reçu une prescription pour des antibiotiques et des stéroïdes dans un centre de soins ambulatoires en septembre 2015 en raison de symptômes d’infection des voies respiratoires supérieures. Quatre mois plus tard, elle a eu un malaise et des fièvres intermittentes accompagnées d’anémie et d’insuffisance rénale aiguë. Un examen approfondi a révélé des cultures sanguines négatives mais les symptômes étaient évocateurs d’une amyloïdose, c’est pourquoi un traitement à base de stéroïdes systémiques a été amorcé avant de référer la patiente à notre institution.
Dans notre centre, une échocardiographie transœsophagienne et une imagerie par résonance magnétique cardiaque ont révélé une prothèse aortique mécanique normale accompagnée d’un abcès au niveau de l’anneau aortique. Un traitement empirique d’antibiotiques a été amorcé chez la patiente pour traiter une endocardite. Une biopsie rénale a révélé une néphrite interstitielle avec un granulome, et on lui a à nouveau prescrit des stéroïdes à fortes doses. L’état de la patiente a continué à se détériorer et sa fonction rénale à empirer, et une pancytopénie est apparue, laquelle a nécessité des transfusions quotidiennes d’érythrocytes et de plaquettes.
Trois semaines après son hospitalisation, ses cultures sanguines étaient positives pour le Mycobacterium chimera et un régime posologique de quatre médicaments, soit la rifampicine, la rifabutine, l’éthambutol et la clarithromycine a été mis en place. Ce traitement a entraîné des améliorations cliniques spectaculaires.
Conclusion
Les générateurs thermiques utilisés pendant la circulation extracorporelle fabriqués par LivaNova (Munich, Allemagne) avant septembre 2014 ont été associés à la M. Chimera, une infection latente qui peut être précipitée et devenir une infection disséminée dans les cas d’immunosuppression liée à l’utilisation de stéroïdes.
Similar content being viewed by others
Infection is a major complication after cardiac surgery. Risks such as the use of central venous catheters, implantation of foreign material, hypothermia, and allogeneic blood transfusions can all increase the potential for nosocomial infection.1,2,3,4 A subject of interest involves contaminants in the heater-cooler units (HCUs) of the cardiopulmonary bypass circuit, which may serve as a reservoir and source of infection in the operating room. The HCU functions as a thermoregulator by using reservoirs of filtered tap water as a thermic transfer medium to cool the cardioplegia solution and to alternately cool and warm the patient’s blood. Non-tuberculous mycobacterium (NTM) is a rare pathogen that can be transmitted via such a moisture-rich environment. Heater-cooler units were first suspected to be the cause of NTM infection in 20025 and have recently gained global interest because of newly reported cases.6,7
The purpose of this report is to present the case of an NTM aortic valve abscess that appeared five years after mechanical valve replacement. We postulate that the infection - likely caused by a mycobacterium harbored in an HCU - may have been exacerbated by subsequent steroid-induced immunosuppression. In addition, we discuss recommendations on the prevention and diagnosis of HCU infections that, in addition to vigilant HCU cleaning, must also include provider and patient awareness of the risk of NTM infection.
Case report
A 63-yr-old woman with a past medical history of aortic coarctation repair in 1963 and mechanical aortic valve replacement in 2010 presented to an outpatient care facility in September 2015 with symptoms of an upper respiratory tract infection. She was prescribed antibiotics and systemic steroids, which improved her symptoms. In January 2016, she presented with malaise and intermittent fevers and was discovered to have anemia and acute kidney dysfunction but no obvious infection. An extensive evaluation was performed, including bone marrow biopsy, which showed granulomas and a fatty aspirate suggestive of amyloidosis. The patient was started on systemic steroids for presumed sarcoidosis and amyloidosis and was referred to us for further evaluation.
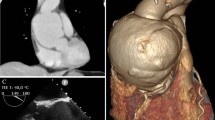
At our institution, transesophageal echocardiography (TEE) performed to investigate for potential prosthetic valve endocarditis showed a normally functioning St. Jude mechanical aortic prosthesis. It also revealed aortic root thickening with cavitation consistent with an aortic root abscess and punctate thickening of the mitral valve suggesting possible previous endocarditis (Figure) (Electronic Supplementary Material, Videos 1 and 2). The patient was started on vancomycin and ceftriaxone. Subsequent cardiac magnetic resonance imaging confirmed an aortic root abscess and a 5-mm area of linear mobile density on the ventricular side of the aortic valve prosthesis. Renal biopsy indicated interstitial nephritis with one granuloma, for which she was again started on high-dose steroids. She continued to deteriorate, however, with worsening renal function and developed pancytopenia requiring daily red blood cell and platelet transfusions.
Transesophageal echocardiographic image (mid-esophageal long axis view) shows a posterior aortic root abscess (white arrow) adjacent to the prosthetic aortic valve, with colour flow Doppler demonstrating communication between the aorta and the abscess cavity. LVOT = left ventricular outflow tract; Asc aorta = ascending aorta
Three weeks after hospitalization, her blood cultures were reported to be positive for mycobacterium, and she was started on a four-drug regimen of rifampin, rifabutin, ethambuton, and clarithromycin with dramatic improvement. Speciation of the organism revealed Mycobacterium chimera (M. chimera). At the time of this report, she was actively being followed by our cardiothoracic surgery team for potential aortic valve explantation, aortic root debridement, and redo tissue aortic valve replacement.
This case is being published with the patient’s consent.
Discussion
Infections after cardiac surgery are associated with both increased hospital readmission and mortality.8,9 In a review of more than 300,000 patients in the Society of Thoracic Surgeons National Cardiac Database, patients with major infections after cardiothoracic surgery were noted to have higher mortality (17.3% vs 3.0%; P < 0.001) and an increased hospital stay of > 14 days (47% vs 5.9%; P < 0.001) compared with patients without major infection.9 Prosthetic valve endocarditis is one such major infection that can lead to valve malfunction, subsequent surgical intervention, and other systemic disease. It occurs in 1-6% of patients with prosthetic valves.10 Non-tuberculous mycobacteria is a known cause of sternal wound infections, prosthetic valve endocarditis, and other post-cardiac surgery related infections.11,12,13,14,15,16 The first cases of NTM endocarditis in 1976 were nosocomial and were associated with porcine valves infected with NTM resistant to the formaldehyde used for sterilization.16
In recent years, clusters of M. chimera cases have been reported in Canada, Europe, and the United States.17 In 2015, six cases were reported from Zurich in thoracic surgery patients who were found to have infection of the intrathoracic prosthesis. The patients presented with fever, weight loss, pancytopenia, renal failure, fatigue, and granulomatous disease (granulomatous hepatitis, endocarditis and myocarditis, nephritis, osteomyelitis).6 At the time, the source of infection was believed to be from aerosolized water droplets from the HCUs because water samples in the HCUs and air samples obtained in the operating room were both positive for the same strain of NTM.7 Subsequently, Germany and The Netherlands reported NTM cases connected to HCUs.7,18 In July 2015, a cluster of invasive NTM was reported in Pennsylvania.19 All reported cases involved a latency period of up to five years after surgery, although the subject of specific precipitant factors for recrudescence of the dormant disease was not addressed.
The recognition of HCU infections in Europe and the United States prompted field investigations by the Centers for Disease Control, and speculation arose that the HCUs’ original manufacturing process may have been the source of contamination. It was found that all of these HCUs were from the same manufacturer - LivaNova (London, UK), previously Sorin Group (Munich, Germany) - which supplied 60% of all HCUs in the United States.20 The company initiated investigations and changed its disinfection and drying process in mid-August 2014. In December 2015, information was released regarding six water samples contaminated with M. chimera, two from known contaminated HCUs, three from new units, and one from a sample taken in the pump assembly area at the manufacturing site.21,22 In light of these new data, the United States Food and Drug Administration and the Centers for Disease Control issued guidelines to cardiac surgery centers to 1) direct the HCU exhaust vent away from the surgical field to mitigate the risk of aerosolizing tank water into the sterile field; 2) use only sterile water that has been passed through a < 0.22 μm filter; 3) strictly adhere to the disinfection instructions of the manufacturer; 4) remain vigilant for infections in patients who had undergone surgery in an operating theatre that used LivaNova HCUs manufactured prior to September 2014; and 5) continue to monitor upcoming reports.20,23 LivaNova continues to encourage proper cleaning and maintenance of machines. It believes that, if these steps are properly followed, the machines “pose minimal risk to NTM transmission”.24
Mycobacteria infections linked to contaminated HCUs pose a diagnostic challenge because the incidence is low and symptoms often do not develop until years after exposure during cardiac surgery. The present case represents a latent NTM likely originating from exposure to a LivaNova HCU manufactured prior to September 2014. Given the chronology of symptom onset after steroid use, we postulate that the repeated episodes of steroid-related immunosuppression25 may have precipitated and exacerbated the patient’s latent infection. Case reports previously have not focused on precipitating factors that activate latent NTM. It is thus of utmost importance that not only must HCUs be vigilantly cleaned according to the manufacturer’s recommendations but also that health care leaders must be continually mindful of new developments regarding NTM infection, its presentations, and its precipitating factors.
References
Le Guillou V, Tavolacci MP, Baste JM, et al. Surgical site infection after central venous catheter-related infection in cardiac surgery. Analysis of a cohort of 7557 patients. J Hosp Infect 2011; 79: 236-41.
Stamos MJ. Lessons learned in intraoperative hypothermia: coming in from the cold. JAMA Surg 2015; 150: 575-6.
Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med 1996; 334: 1209-15.
Horvath KA, Acker MA, Chang H, et al. Blood transfusion and infection after cardiac surgery. Ann Thorac Surg 2013; 95: 2194-201.
Weitkemper HH, Spilker A, Knobl HJ, Korfer R. The heater-cooler unit–a conceivable source of infection. J Extra Corpor Technol. 2002; 34: 276-80.
Sax H, Bloemberg G, Hasse B, et al. Prolonged outbreak of Mycobacterium chimaera infection after open-chest heart surgery. Clin Infect Dis 2015; 61L: 67-75.
Kohler P, Kuster SP, Bloemberg G, et al. Healthcare-associated prosthetic heart valve, aortic vascular graft, and disseminated Mycobacterium chimaera infections subsequent to open heart surgery. Eur Heart J 2015; 36: 2745-53.
Gelijns AC, Moskowitz AJ, Acker MA, et al. Management practices and major infections after cardiac surgery. J Am Coll Cardiol 2014; 64: 372-81.
Fowler VG Jr, O’Brien SM, Muhlbaier LH, Corey GR, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation 2005; 112: I358-65.
Habib G, Thuny F, Avierinos JF. Prosthetic valve endocarditis: current approach and therapeutic options. Prog Cardiovasc Dis 2008; 50: 274-81.
Jarzembowski JA, Young MB. Nontuberculous mycobacterial infections. Arch Pathol Lab Med 2008; 132: 1333-41.
Wallace RJ, Musser JM, Hull SI, et al. Diversity and sources of rapidly growing mycobacteria associated with infections following cardiac surgery. J Infect Dis 1989; 159: 708-16.
Unai S, Miessau J, Karbowski P, Bajwa G, Hirose H. Sternal wound infection caused by Mycobacterium chelonae. J Card Surg 2013; 28: 687-92.
Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria. Clin Infect Dis 2001; 33: 1363-74.
Jonsson G, Rydberg J, Sturegard E, Christensson B. A case of Mycobacterium goodii prosthetic valve endocarditis in a non-immunocompromised patient: use of 16S rDNA analysis for rapid diagnosis. BMC Infect Dis 2012; 12: 301.
Laskowski LF, Marr JJ, Spernoga JF, et al. Fastidious mycobacteria grown from porcine prosthetic-heart-valve cultures. N Engl J Med 1977; 297: 101-2.
Laframboise K. 6 Quebec hospitals warn heart surgery patients of potential infection risk. CBC News. Available from URL: http://www.cbc.ca/news/canada/montreal/montreal-heart-institute-1.3832374 (accessed December 2016).
Achermann Y, Rossle M, Hoffmann M, et al. Prosthetic valve endocarditis and bloodstream infection due to Mycobacterium chimaera. J Clin Microbiol 2013; 51: 1769-73.
Sommerstein R, Jenni H, Carrel T, Marschall J. Cardiac surgery, nosocomial infection, and the built environment. J Hosp Infect 2016; 93: 240-1.
Center for Disease Control and Prevention. Contaminated Devices Putting Open-Heart Surgery Patients at Risk. Available from URL: http://www.cdc.gov/media/releases/2016/p1013-contaminated-devices-.html (accessed December 2016).
Haller S, Holler C, Jacobshagen A, et al. Contamination during production of heater-cooler units by Mycobacterium chimaera potential cause for invasive cardiovascular infections: results of an outbreak investigation in Germany, April 2015 to February 2016. Euro Surveill 2016. DOI:10.2807/1560-7917.ES.2016.21.17.30215.
Perkins KM, Lawsin A, Hasan NA, et al. Notes from the field: Mycobacterium chimaera contamination of heater-cooler devices used in cardiac surgery - United States. MMWR Morb Mortal Wkly Rep 2016; 65: 1117-8.
U.S. Food and Drug Administration. UPDATE: Mycobacterium chimaera Infections Associated with LivaNova PLC (formerly Sorin Group Deutschland GmbH) Stöckert 3T Heater-Cooler System: FDA Safety Communication. Available from URL: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm520191.htm (accessed December 2016).
Sorin Group. 3T FAQ. Available from URL: http://www.livanova.sorin.com/products/cardiac-surgery/perfusion/hlm/3t (accessed December 2016).
Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 2011; 335: 2-13.
Conflicts of interest
The authors have no potential conflicts of interest including commercial relationships such as consultation and equity interests. Source of funding for this submission is Mayo Clinic, Department of Anesthesiology and Perioperative Medicine. Mayo Clinic does not endorse the products mentioned in this article.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Yi Cai, Kevin Landolfo and Johnathan R. Renew contributed substantially to the conception and design of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video 1 Transesophageal echocardiographic image (mid-esophageal aortic valve short axis view) shows a posterior aortic root abscess adjacent to a previously implanted mechanical aortic prosthesis. Supplementary material 1 (WMV 913 kb)
Video 2 Transesophageal echocardiographic image (mid-esophageal long axis view) shows a posterior aortic root abscess with colour flow Doppler demonstrating communication between the aorta and the abscess cavity. Supplementary material 2 (WMV 885 kb)
Rights and permissions
About this article
Cite this article
Cai, Y., Landolfo, K. & Renew, J.R. Mycobacterium infection from a cardiopulmonary bypass heater-cooler unit in a patient with steroid-induced immunosuppression. Can J Anesth/J Can Anesth 64, 513–516 (2017). https://doi.org/10.1007/s12630-016-0809-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-016-0809-5