Abstract
Purpose
The sum of the volumes of brain tissue, cerebrospinal fluid (CSF), and intracranial blood remain constant. This tenet of the Monroe-Kellie hypothesis is most often considered in the setting of intracranial hypertension, but it can also be applied in the setting of CSF volume depletion. We used this hypothesis to explain a case of failed caudal block in a patient with an iatrogenic CSF leak.
Clinical features
A one-month-old baby (3 kg) born at 35 weeks’ gestation presented for right inguinal hernia repair. His past medical history was significant for arthrogryposis (congenital joint contractures in two or more areas of the body) as well as ongoing apneic episodes that required continuous positive airway pressure therapy and neonatal intensive care. An ultrasound confirmed caudal block was completed and within five minutes of the procedure, the patient’s heart rate increased, with an accompanying slight increase in T-wave amplitude. Pinch tests revealed anesthesia to the feet bilaterally but insufficient anesthesia to the abdomen. The surgery was delayed but successfully completed under general anesthesia the following week. Magnetic resonance imaging of the brain and spine following the surgery showed a significant CSF leak with engorgement of the epidural venous plexus along the entire spine. These findings were consistent with a CSF leak likely secondary to a prior lumbar puncture (at age 13 days) that was part of the investigation of his respiratory issues.
Conclusions
The possible mechanism of this failed caudal block was high systemic absorption of anesthetic given the epidural venous plexus engorgement thus leaving less anesthetic acting within the CSF and on the exiting spinal nerves. Decreased CSF flow in the thecal sac might also have contributed, as might dilution of the remaining local anesthetic caused by large amounts of leaking CSF within the epidural space.
Résumé
Objectif
La somme des volumes du tissu cérébral, du liquide céphalo-rachidien (LCR) et du sang intracrânien reste constante. Cette doctrine de l’hypothèse de Monroe-Kellie est le plus souvent retenue dans le contexte de l’hypertension intracrânienne, mais elle peut également s’appliquer dans le cadre d’une déplétion du volume de LCR. Nous avons utilisé cette hypothèse pour expliquer un cas d’échec de bloc caudal chez un patient présentant une fuite iatrogénique de LCR.
Caractéristiques cliniques
Un bébé âgé d’un mois (3 kg) né à 35 semaines de grossesse a été vu pour correction chirurgicale d’une hernie inguinale droite. Ses antécédents médicaux étaient significatifs : arthrogrypose (contractures articulaires congénitales dans au moins deux régions du corps) et épisodes d’apnée continus nécessitant une thérapie par pression positive continue des voies respiratoires et soins néonatals intensifs. Une échographie a confirmé la réalisation du bloc caudal, mais la fréquence cardiaque du patient a augmenté dans les 5 minutes du début de l’intervention avec une légère augmentation associée de l’amplitude de l’onde T. Des tests de pincement ont révélé que l’anesthésie était correcte au niveau des deux pieds, mais insuffisante au niveau de l’abdomen. L’intervention chirurgicale a été retardée et réalisée avec succès la semaine suivante sous anesthésie générale. Après la chirurgie, une imagerie par résonance magnétique du cerveau et de la colonne vertébrale a mis en évidence une fuite de LCR significative avec engorgement des plexus veineux périduraux sur toute la longueur du rachis. Ces constatations étaient compatibles avec une fuite de LCR probablement secondaire à une ponction lombaire antérieure (à l’âge de 13 jours) réalisée pour l’enquête diagnostique de ses problèmes respiratoires.
Conclusions
Le mécanisme de cet échec de bloc caudal était possiblement une absorption systémique importante de l’anesthésique compte tenu de l’engorgement du plexus veineux péridural, laissant ainsi moins d’anesthésique actif dans le LCR et au contact des racines des nerfs rachidiens. La diminution du débit de LCR dans le sac thécal peut y avoir également contribué, tout comme la dilution de l’anesthésique local restant provoquée par la grande quantité de LCR ayant fui dans l’espace péridural.
Similar content being viewed by others
The Monroe-Kellie hypothesis states that the total volumes of brain tissue, cerebrospinal fluid (CSF), and intracranial blood remain constant. Any change in one of these volumes leads to a reciprocal change in the others. This tenet is most often used in the setting of intracranial hypertension, but it can also be applied in the presence of CSF volume depletion. The purpose of this case report is to use this hypothesis to explain a case of failed caudal block thought to be secondary to physiologic changes that occurred with CSF leakage.
Clinical case
A one month-old baby (3 kg) born at 35 weeks’ gestation presented for right inguinal hernia repair. His past medical history was significant for arthrogryposis (congenital joint contractures in two or more areas of the body) with bilateral ankle and hand contractures and hip dysplasia. At birth, he made no spontaneous respiratory efforts and so was intubated for five days and extubated to bilevel positive airway pressure. Numerous attempts at weaning him to high-flow nasal prongs because of ongoing apneic episodes that resulted in significant hypoxemia and bradycardia failed. He remained on a continuous positive airway pressure (CPAP) device with settings of +6 cm H2O and FiO2 0.21. Biochemical and hematologic investigations, chest radiography, and echocardiography were all within normal limits. A full septic workup including a lumbar puncture at 13 days of age revealed normal CSF. He remained in the neonatal intensive care unit because of continuing respiratory issues.
On the day of the operation, tetracaine hydrochloride gel (AmetopTM, Smith-Nephew, Mississauga, ON, Canada) was applied over the sacrum, and the baby was transferred to the operating room. Following placement of standard anesthetic monitors,1 he was placed in the right lateral decubitus position and remained on CPAP with the FiO2 at 0.6 during the procedure. A caudal block using a 22G angiocatheter (BD Angiocatheter™, Becton, Dickinson and Co., Franklin Lakes, NJ, USA) was completed using an aseptic technique. Ultrasonography confirmed cannula placement in the caudal space and verified dilation of the caudal epidural space during intermittent injection of the local anesthetic. There was no blood or clear fluid noted from the cannulas prior to attaching the 3-mL Luer-Lock syringe. Aspiration was negative, so 3 mL of local anesthetic (2 mL of 0.25% bupivacaine with 1:200,000 epinephrine plus 1 mL of 1% lidocaine) was slowly injected over two minutes. The patient was then repositioned in the supine position. Approximately three minutes following injection of the anesthetic mixture, the patient’s heart rate increased from 170 beats·min−1 to a maximum of 230 beats·min−1 before dropping to 215 beats·min−1 over one to two minutes. The T-wave amplitude also increased slightly, but there were no other changes in the PR interval or QRS morphology. His blood pressure slightly decreased (from 108/58 mmHg to 95/64 mmHg) following the caudal block. Muscular twitches in the upper and lower limbs were noted, but there was no seizure or loss of consciousness. The resuscitation cart, including Intralipid solution, was brought into the operating room, but the baby remained stable with sinus tachycardia and no resuscitation was required.
A pinch test to his feet performed 15 min after the caudal injection revealed no response, but his abdomen was not anesthetized. The surgeon attempted additional pinch tests at 20 and 25 min, and it was clear that the caudal anesthetic had failed. A decision was made to postpone the case because we could not use additional local anesthetic and the clinical picture (i.e., increased heart rate, T-wave changes, inadequate abdominal anesthesia) suggested possible intravascular injection of the local anesthetic mixture.
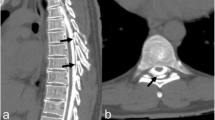
In the interim, an ultrasound scan of the spine was performed to assess for any anatomic abnormalities that may have contributed to the failed caudal block. As seen in Fig. 1, several intradural leptomeningeal linear structures of unknown etiology were noted. These “septum-like” structures, which were associated with a thickened dura (possibly due to mild arachnoid inflammation) at the lumbar level, were thought to be secondary to the previously performed lumbar puncture (at 13 days of age).
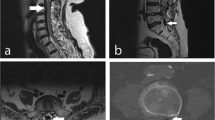
One week later, we successfully completed the patient’s hernia repair under combined general anesthesia and ultrasound-guided ilioinguinal and iliohypogastric nerve blocks. Immediately following the procedure, he underwent magnetic resonance imaging (MRI) of the brain and spine for evaluation of possible developmental abnormalities as well as further characterization of the thickened dura noted on the ultrasound scan. The MRI findings showed a significant CSF leak (Fig. 2) and engorgement of the epidural venous plexus (Fig. 3a, 3b) along the entire spine with increased prominence at the lumbosacral level. These radiological findings were consistent with a CSF leak secondary to the previous lumbar puncture. Another MRI finding consistent with CSF depletion was dural thickening in the spine.2
a Sagittal fat-saturated T1-weighted post-gadolinium magnetic resonance image (MRI) of the spine showing significant epidural vein engorgement (white arrows) at the lumbosacral level. b Axial fat-saturated T1-weighted post-gadolinium MRI of the spine shows engorged epidural veins at the ninth thoracic vertebral level. Both arrows point to engorged epidural veins at the T9 vertebral level. The white arrow shows a hyperdense (bright “V”-like) structure representing venous contrast caused by low venous flow. The yellow arrow points to engorged, hypodense (dark, round) epidural veins with no evidence of contrast because of the high venous flow
Discussion
The MRI findings of this patient demonstrate the principles of the Monr-Kellie hypothesis – that is, the sum of the volumes of brain tissue, CSF, and intracranial blood is constant.2 Although the Monroe-Kellie hypothesis is most often discussed in the clinical setting of intracranial hypertension, it can also be applied for the reverse situation of CSF volume depletion in the setting of a CSF leak. Interestingly, there are also CSF changes that take place at the spinal level. Within the spinal canal, there is epidural space between the dura and the fibro-osteal canal that is filled with fatty areolar tissue and an epidural venous plexus.2 Even mild dural collapse caused by a CSF leak will lead to epidural venous hyperemia and engorgement.3,4 Hasiloglu et al. used magnetic resonance CSF flow studies to evaluate CSF physiology in patients with spontaneous intracranial hypotension (SIH). They found that the CSF volume flowing toward the third ventricle, CSF flow toward the fourth ventricle, absolute cardiac stroke volume, peak systolic flow velocity, and peak diastolic velocity were significantly decreased in SIH patients compared with those in control subjects.5 Hence, we suggest that our patient’s physiologic spinal CSF flow decreased secondary to a CSF leak, which may have contributed to decreased drug diffusion into the dural sac.
The precise location of neural blockade from epidural anesthetics is not well delineated. In fact, the blockade may affect the spinal nerves in the epidural space, the spinal nerve rootlets within the CSF, or the spinal cord. Current thinking suggests that early nerve block occurs as an extradural radicular block, with a subdural spinal block occurring later.6,7
We speculate that the etiology of the failed caudal block could have been multifactorial in this patient. We considered one of the most likely mechanisms to be high systemic absorption of the injected anesthetic due to significant epidural venous plexus engorgement. The engorged epidural veins may have then led to rapid uptake of the local anesthetic, evidenced by the tachycardia and peaked T waves. This situation would leave less anesthetic drug acting on the exiting spinal nerves and in the CSF. Although the caudal block was confirmed by ultrasonography, and we had negative aspiration prior to injecting the local anesthetic, there is also the possibility that inadvertent intravascular injection caused the failed block. In addition, engorgement of the intradural veins and decreased CSF flow in the thecal sac may have contributed to the failed block. Although we performed a limited sensory examination at the time, the block seemed to be limited to the sacral area, with no evidence of low thoracic or high lumbar anesthesia. Lastly, the large amount of CSF in the epidural space may have diluted the remaining local anesthetic that had not been absorbed into the spinal canal and nerves because of the decreased CSF flow and the prominent veins.8,9
Pediatric studies suggest that children presenting for urologic or general surgical procedures at a young age have an increased incidence of spinal abnormalities, including a low conus medullaris and tethered cord when compared with the general population.10,11 It has previously been advocated that, because of this association, preoperative spinal imaging should be considered prior to administering a caudal anesthetic to children presenting for these types of procedures at a young age.10 Our case provides further evidence that supports the use of pre-procedure spinal imaging in neonates and infants who have recently undergone a lumbar puncture and who will be receiving a spinal or caudal anesthetic. Although ultrasonography and fast MRI have some limitations, they could provide important information without the need for general anesthesia. This information could influence the subsequent choice of regional anesthetic technique in these children.
References
Merchant R, Chartrand D, Dain S, et al. Guidelines to the practice of anesthesia – revised edition 2016. Can J Anesth 2016; DOI:10.1007/s12630-015-0470-4.
Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology 2001; 56: 1746-8.
Burtis MT, Ulmer JL, Miller GA, Barboli AC, Koss SA, Brown WD. Intradural spinal vein enlargement in craniospinal hypotension. AJNR Am J Neuroradiol 2005; 26: 34-8.
Fettes PD, Jansson JR, Wildsmith JA. Failed spinal anaesthesia: mechanisms, management, and prevention. Br J Anaesth 2009; 102: 739-48.
Hasiloglu ZI, Albayram S, Gorucu Y, et al. Assessment of CSF flow dynamics using PC-MRI in spontaneous intracranial hypotension. Headache 2012; 52: 808-19.
Urban BJ. Clinical observations suggesting a changing site of action during induction and recession of spinal and epidural anesthesia. Anesthesiology 1973; 39: 496-503.
Carvalho JC, Khemka R, Loke J, Tsui BC. Low-dose intrathecal local anesthetic does not increase the threshold current for the epidural stimulation test: a prospective observational trial of neuraxial analgesia in labouring women. Can J Anesth 2015; 62: 265-70.
Carpenter RL, Hogan QH, Liu SS, Crane B, Moore J. Lumbosacral cerebrospinal fluid volume is the primary determinant of sensory block extent and duration during spinal anesthesia. Anesthesiology 1998; 89: 24-9.
Hogan QH. Lumbar epidural anatomy: a new look by cryomicrotome section. Anesthesiology 1991; 75: 767-75.
Koo BN, Hong JY, Song HT, Kim JM, Kil HK. Ultrasonography reveals a high prevalence of lower spinal dysraphism in children with urogenital anomalies. Acta Anaesthesiol Scand 2012; 56: 624-8.
Kim SM, Change HK, Lee MJ, et al. Spinal dysraphism with anorectal malformation: lumbosacral magnetic resonance imaging evaluation of 120 patients. J Pediatr Surg 2010; 45: 769-76.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Erin Bruce, Adam O. Spencer, and Mehmet Sait Albayram contributed substantially to all aspects of this manuscript, including conception and design; acquisition, analysis, and interpretation of data; and drafting the article.
Rights and permissions
About this article
Cite this article
Bruce, E., Spencer, A.O. & Albayram, M.S. Failed caudal block due to physiologic changes associated with a cerebrospinal fluid leak: a case report. Can J Anesth/J Can Anesth 63, 603–607 (2016). https://doi.org/10.1007/s12630-016-0584-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-016-0584-3