Abstract
Purpose
The efficacy of myocardial conditioning strategies is compromised in patients with advanced age, diabetes, or low ejection fraction. We conducted a single-centre parallel-arm blinded randomized-controlled trial to determine whether propofol provides perioperative myocardial protection.
Methods
Patients enrolled in this study were scheduled for primary aortocoronary bypass surgery utilizing normothermic cardiopulmonary bypass (CPB) with blood cardioplegia. The participants were stratified by diabetic status and left ventricular ejection fraction and randomly assigned to receive either an elevated dose of propofol –previously associated with experimental cardioprotection– or an isoflurane preconditioning regime. The primary endpoint was the coronary sinus (CS) concentration of 15-F2t-isoprostane (isoP). Secondary endpoints included in-hospital low cardiac output syndrome (LCOS) and major adverse cardiac events, 12- and 24-hr CS cardiac troponin I (cTnI) release, and myocardial B-cell lymphoma 2 (Bcl-2) protein expression.
Results
Data were analyzed from 125 of 137 randomized participants. Participants receiving propofol experienced a greater mean (SD) increase from baseline in CS 15-F2t-isoP levels compared with those receiving isoflurane [26.9 (10.9) pg·mL−1 vs 12.1 (10.4) pg·mL−1, respectively; mean difference, 14.8; 95% confidence interval (CI), 11.0 to 18.6; P < 0.001] but a decreased incidence of LCOS (20.9% vs 57.1%, respectively; relative risk [RR],0.37; 95% CI, 0.22 to 0.62; P < 0.001). The incidence of LCOS was similar between groups in participants without type 2 diabetes mellitus (DM2) (P = 0.382) but significantly decreased in the propofol DM2 subgroup compared with the isoflurane DM2 subgroup (17.9% vs 70.3%, respectively; RR, 0.26; 95% CI, 0.13 to 0.52; P < 0.001). Propofol was associated with an increase in myocardial Bcl-2 protein expression (P = 0.005), a lower incidence of a CS cTnI threshold for myocardial infarction (P = 0.014), and fewer heart failure events (P < 0.001).
Conclusion
Propofol may be a preemptive intraoperative cardioprotectant for patients with DM2 under conditions of normothermic CPB and blood cardioplegic arrest. The study is registered at www.clinicaltrials.gov (NCT00734383) and www.controlled-trials.com (ISRCTN70879185).
Résumé
Objectif
L’efficacité des stratégies de conditionnement myocardique est compromise chez les patients âgés ainsi que chez ceux atteints de diabète ou présentant une fraction d’éjection faible. Nous avons réalisé une étude randomisée contrôlée unicentrique à bras parallèles et en aveugle afin de déterminer si le propofol procurait une protection myocardique en période périopératoire.
Méthode
Les patients enrôlés dans cette étude devaient subir une chirurgie de pontage aorto-coronarien primaire avec circulation extracorporelle (CEC) normothermique et cardioplégie sanguine. Les participants ont été stratifiés par statut diabétique et fraction d’éjection ventriculaire gauche, puis aléatoirement répartis en deux groupes, dont l’un recevrait une dose élevée de propofol – un agent précédemment associé à une cardioprotection expérimentale – et l’autre un régime de préconditionnement à l’isoflurane. Le critère d’évaluation principal était la concentration dans le sinus coronaire (SC) de 15-F2t-isoprostane (isoP). Les critères d’évaluation secondaires comprenaient la survenue d’un syndrome de bas débit cardiaque (SBDC) pendant le séjour hospitalier et les complications cardiaques majeures, la libération de troponine I cardiaque (cTnI) du SC à 12 et 24 h, et l’expression protéinique du lymphome 2 à cellules B (Bcl-2) myocardique.
Résultats
Les données de 125 des 137 patients randomisés ont été analysées. Les participants ayant reçu du propofol ont subi une augmentation moyenne (ÉT) plus importante depuis les valeurs de base en matière de niveaux au SC de 15-F2t-isoP par rapport aux patients ayant reçu de l’isoflurane [26,9 (10,9) pg·mL−1 vs 12,1 (10,4) pg·mL−1, respectivement; différence moyenne, 14,8; intervalle de confiance (IC) 95 %, 11,0 à 18,6; P < 0,001], mais une incidence moindre de SBDC (20,9 % vs 57,1 %, respectivement; risque relatif [RR], 0,37; IC 95 %, 0,22 à 0,62; P < 0,001). L’incidence de SBDC était semblable dans les deux groupes chez les participants qui n’étaient pas atteints de diabète de type 2 (DT2) (P = 0,382), mais significativement réduite dans le sous-groupe DT2 propofol par rapport au sous-groupe DT2 isoflurane (17,9 % vs 70,3 %, respectivement; RR, 0,26; IC 95 %, 0,13 à 0,52; P < 0,001). Le propofol a été associé à une augmentation de l’expression protéinique du Bcl-2 myocardique (P = 0,005), une incidence moindre de seuil de cTnI du SC pour un infarctus du myocarde (P = 0,014), et moins d’épisodes d’insuffisance cardiaque (P < 0,001).
Conclusion
Le propofol pourrait constituer un cardioprotecteur peropératoire préventif pour les patients atteints de DT2 sous CEC normothermique et en arrêt cardioplégique sanguin. Cette étude est enregistrée au www.clinicaltrials.gov (NCT00734383) et au www.controlled-trials.com (ISRCTN70879185).
Similar content being viewed by others
Ischemia-reperfusion injury (IRI) is a major factor in the development of myocardial infarction and heart failure events following aortocoronary bypass (ACBP) surgery.1 Patients with advanced age, diabetes, or low preoperative ventricular ejection fraction are at a particularly high risk for these complications.1–3 Low cardiac output syndrome (LCOS) secondary to myocardial stunning or necrosis increases postoperative mortality by as much as ten to 17-fold.3 It is essential to develop a preemptive intraoperative therapeutic strategy to counter these effects.
Since the discovery of ischemic preconditioning by Murry et al. nearly 30 years ago, efforts have focused on strategies that increase the tolerance of the myocardium to IRI.4 Unfortunately, pharmacologic or physical conditioning strategies are not effective in the aged or chronic diabetic heart due to corruption of cardioprotective signalling pathways and mitochondrial dysfunction.5–11 As oxidative stress is a major factor in the pathophysiology of IRI, propofol, an anesthetic and phenolic antioxidant, could be a therapeutic alternative.12,13Nevertheless, meta-analyses of clinical trials show reduced indices of myocardial injury and dysfunction with inhalational agents compared with propofol anesthesia (2-4 µg·mL−1) in low-risk ACBP surgery.14,15 A better approach to achieve cardioprotection might be to apply propofol in higher concentrations solely during the ischemia-reperfusion interval.16
Applying propofol just before, during, and briefly following ischemia-reperfusion in rat heart models decreases release of plasma free 15-F2t-isoprostane,17,18 a biomarker of oxidative stress. It exacerbates myocardial IRI, producing left ventricular dysfunction directly via receptor-induced coronary artery vasoconstriction.19 Furthermore, we previously found that propofol upregulates anti-apoptotic B-cell lymphoma 2 (Bcl-2) gene and protein expression in cardiomyoblasts, allowing them to withstand subsequent oxidative challenge.20 B-cell lymphoma 2 sequesters pro-apoptotic BAX, a protein that promotes mitochondrial pore opening. This attenuates free radical release, death sequence activation, and IRI.21 This could decrease 15-F2t-isoprostane generation during reperfusion associated with postoperative ventricular dysfunction in patients.22
This report presents the effects of a propofol infusion designed to achieve drug concentrations in patients during cardiopulmonary bypass (CPB) that alleviate experimental oxidative injury.23 Accordingly, in this study, we investigated the concentration and time-dependent effects of propofol (whole blood concentration 4-12 µg·mL−1) compared with isoflurane on cyto- and cardioprotective measures of oxidative injury.16,17 We hypothesized that propofol would decrease 15-F2t-isoprostane release and, as a result, reduce the incidence of LCOS. We further explored concomitant myocardial Bcl-2 expression as it may affect or be affected by 15-F2t-isoprostane release. Lastly, these endpoints and outcomes were differentially investigated in patients with or without type 2 diabetes mellitus (DM2).
Methods
PRO-TECT II is a single-centre phase 2 blinded randomized-controlled trial.24 The study was approved (July 2005) by the University of British Columbia Clinical Research Ethics Board and conforms to the principles outlined in the Declaration of Helsinki.
Participants
We enrolled adult patients at the Vancouver General Hospital after obtaining their written informed consent. Patients were eligible for participation if they were 18-80 yr of age and scheduled for elective primary ACBP surgery that entailed revascularization of three or more coronary arteries with cardiopulmonary bypass (CPB) and an anticipated aortic cross-clamp (ACC) time of at least 60 min. Patients were ineligible if they had type 1 diabetes mellitus (DM), coexisting valvular heart disease, an acute or evolving myocardial infarction within seven days of surgery, or a history of hypersensitivity to propofol (or its various components). Given the link between inflammation and oxidative stress, patients taking nonsteroidal anti-inflammatory drugs (including acetylsalicylic acid) or antioxidants within five to seven days of surgery were excluded to avoid potential confounding of our primary outcome variable.
Randomization and blinding
Participants were randomly allocated to either propofol (cardioprotection) or isoflurane (preconditioning) using a computer-generated random number table. Randomization was accomplished with permuted blocks of four or six, stratified by history of DM (no DM or DM2) and by the preoperative left ventricular ejection fraction based on angiography – i.e., normal ≥ 45% or low < 45%.
A study anesthesiologist or fellow initiated the study intervention, leaving the patients, surgeons, investigators, and attending anesthesiologist blinded to the experimental patient therapy. Blinding was facilitated by covering anesthetic vaporizers with opaque drapes, turning off the end-tidal anesthetic agent readout, and mimicking use of propofol in the isoflurane group by infusing Intralipid® 20% lipid emulsion (Frensius Kabi, Bad Homburg, DE, Germany) on the same side as the patient’s intravenous catheter into a receptacle concealed from view. During CPB, the study anesthesiologist directed an unblinded perfusionist regarding anesthetic management. Following separation from CPB, draping was removed and the blinded anesthesiologist resumed care. Staff providing postoperative care were unaware of study group allocation.
Procedures
Intravenous and arterial cannulae were inserted prior to anesthetic induction (fentanyl 10-15 µg.kg−1 iv, midazolam 0.15-0.25 mg.kg−1 iv, and sodium thiopental 1-2 mg.kg−1 iv) and muscle relaxation (rocuronium 1-1.5 mg.kg−1 iv). Anesthesia was maintained with isoflurane 0.5-1.5% (end-tidal) while central venous and pulmonary artery catheterization and transesophageal echocardiography were performed.
Following heparinization, administration of isoflurane to the propofol group was discontinued for ten minutes. At this time, a bolus of propofol 1 mg.kg−1 iv was administered, followed by an infusion of propofol 120 µg.kg−1.min−1 iv administered prior to aortic cannulation and maintained until 15 min after release of the ACC (i.e., reperfusion) (Fig. 1). Participants allocated to the isoflurane group received 2.5% isoflurane (end-tidal) for ten minutes before CPB, followed by isoflurane 0.5-1.0% (end-tidal) during CPB and 15-min reperfusion.
A retrograde coronary sinus (CS) catheter for blood sampling was placed prior to ACC. Nonpulsatile CPB was conducted at 34-37°C after retrograde autologous priming to maintain an intraoperative hematocrit of 0.25- 0.27. Cardioplegia was induced and maintained with intermittent antegrade warm or cold 8:1 and then warm 64:1 blood cardioplegia as per surgeon preference. An arterial conduit was performed to the left anterior descending coronary artery, followed by sequential arterial or saphenous vein grafting as required.
An intravenous infusion of insulin was administered to achieve perioperative glucose levels < 10 mmol.L−1 according to hospital guidelines. Following the study intervention, anesthesia maintenance, sedation, and analgesia were conducted according to routine clinical practice. Echocardiography and pulmonary artery catheter measurements guided the use of fluids and vasoactive drugs for separation from CPB and in the intensive care unit.
Sampling and analysis
Arterial and CS blood samples were drawn prior to ACC and at five minutes after reperfusion. A central venous blood specimen was collected at 15 min after reperfusion. In a small subset of patients (n = 8), cardioplegia samples were drawn from delivery tubing at the midpoint of surgery to determine if the study intervention produced blood cardioplegia enriched with propofol. Right atrial biopsies taken after aortic cannulation and at 15 min after reperfusion were flash frozen in liquid nitrogen and stored along with the blood specimens at -80°C for a subsequent analysis of plasma free 15-F2t-isoprostane (i.e., the primary study outcome), troponin I (TnI), propofol concentration, and myocardial Bcl-2 protein expression.
Plasma free 15-F2t-isoprostane (hereafter denoted as “15-F2t-isoprostane”) was quantitatively analyzed using immunoaffinity purification followed by liquid chromatography-mass spectrometry analysis.25 A prior in vitro study from our laboratory determined that the normothermic heart was a major source of 15-F2t-isoprostane.17,18,26 Mean (SD) arterial 15-F2t-isoprostane measurements were lower than concurrent CS measurements [30.1 (18.9) vs 94.0 (32.1) pg.mL−1, respectively] in samples from the first ten participants that were analyzed without unmasking the allocation. Based on these results, the primary endpoint was modified with subsequent analyses based on only CS 15-F2t-isoprostane levels.
Troponin I concentration in plasma was measured ten minutes prior to CPB and five minutes, 12 hr, and 24 hr after reperfusion by our central hospital laboratory (Siemens Vista analyzer, Siemens Healthcare Diagnostics Ltd, Camberley, UK). Whole blood propofol concentrations in cardioplegia and in central venous blood were determined by capillary electrophoresis as previously reported.27 Myocardial Bcl-2 protein expression in the atrial biopsies was determined by immunoblotting as previously described.28
Clinical outcomes
Participants were diagnosed to have LCOS if they required dopamine or dobutamine > 4 µg.kg−1.min−1 iv, epinephrine or norepinephrine > 0.04 µg.kg−1.min−1 iv, or milrinone ≥ 0.125 µg.kg−1.min−1 iv and/or intra-aortic balloon pump for > 30 min within the first six hours of reperfusion. These treatments help to maintain systolic blood pressure > 90 mmHg and cardiac index > 2.1 L.min−1.m−2 following optimization of heart rate, preload, and afterload.3 The diagnosis was excluded when norepinephrine was used to treat low systemic vascular resistance in the presence of a normal or elevated cardiac index or when echocardiography identified non-cardiac causes of hemodynamic instability.3
Major adverse cardiac events (MACE) were documented as defined by the 2014 American College of Cardiology/American Heart Association cardiovascular endpoints for clinical studies: death, myocardial infarction, unstable angina, transient ischemic attack or stroke, heart failure event (i.e., prolonged LCOS > 24 hr or postoperative congestive heart failure), percutaneous or peripheral vascular intervention.29
Statistical methods
Using prior results for arterial 15-F2t-isoprostane measured by enzyme-linked immunoassay, assuming a mean (SD) 15-F2t-isoprostane of 150 (120) pg.mL−1, a two-sided type I error rate of 0.05, and power of 0.80, 144 participants (36 per stratum - i.e., type 2 DM or no DM, normal or low left ventricular ejection fraction per group) were needed to detect an anticipated relative difference of 50% between groups.24
Data analysis followed the intention-to-treat principle. Categorical data were summarized as counts and percentages. Normal and skewed continuous data were summarized as mean (SD) or median (interquartile range [IQR]), respectively. The Mann-Whitney U test was used for intergroup comparisons of 15-F2t-isoprostane levels. Fisher’s exact test was used for intergroup comparisons, and the relative risk (RR), absolute risk reduction, and their respective 95% confidence intervals (CI) were determined. Subgroup analyses of LCOS episodes based on diabetic status were planned a priori. Coronary sinus cardiac troponin I (CS cTnI) levels were analyzed with the Kruskal-Wallis test. We excluded spurious TnI values from participants without LCOS or MACE indicative of rapid CS sampling or associated with surgical manipulation, cardiac tamponade, or pericarditis. Results were explored in comparison with prognostic thresholds for intraoperative myocardial damage (CS cTnI ≥ 0.5 µg.L−1) and infarct-related events (CS cTnI ≥ 0.94 µg.L−1 and systemic cTnI > 6.93 µg.L−1 in 24 hr).30–32 Post/Pre-CPB Bcl-2 ratios were analyzed using the unpaired two-tailed Student’s t test. A two-sided P < 0.025 was considered statistically significant. Analyses were performed with SPSS® program for Windows Version 15 (SPSS Inc, Chicago, IL, USA) or GraphPad Prism Version 6 (GraphPad Software, San Diego, CA, USA).
Results
During August 2005 to June 2011, 402 patients were screened for eligibility, 148 patients consented to participate in the study, and 137 participants were randomized to propofol cardioprotection (n = 69) or isoflurane preconditioning (n = 68) (Fig. 2). The study was discontinued before the planned target enrolment was reached due to local recruitment for a competing study as well as the unavailability of thiopental. Twelve participants (seven in the propofol group and five in the isoflurane group) were excluded due to a temporary moratorium on institutional research or an unanticipated change of surgery. One hundred twenty-five participants – propofol (n = 62) or isoflurane (n = 63) – were included in the intention-to-treat analysis. One participant in the propofol group developed prolonged pre-bypass ischemia and instability and received only isoflurane (Fig. 2).
Flow diagram of patient enrolment, allocation, follow-up, and analysis. The administrative moratorium refers to a period of institutional review when collection of human specimens was suspended for all clinical trials at the study site. CABG = coronary artery bypass graft surgery; ITT = intention-to-treat
Patient characteristics and medication use were similar between groups (Table 1). Procedural complexity was comparable except for increased use of right internal mammary artery (P = 0.024) or bilateral internal mammary artery grafting (P = 0.009) in the propofol group (Table 1). No 15-F2t-isoprostane data were lacking. Cardiac troponin I data were excluded or missing in 10/125 patients (8%) at reperfusion, 16/125 patients (12.8%) at 12 hr, and 32/125 patients (25.6%) at 24 hr due to early discharge from the intensive care unit. B-cell lymphoma 2 data were lacking in 19/125 patients (15%). No data were lacking in patients experiencing LCOS or MACE.
At baseline, i.e., prior to CPB, the median [IQR] CS 15-F2t-isoprostane was 60.6 [39.7-94.0] pg.mL−1 in the propofol group vs 53.6 [37.6-76.9] pg.mL−1 in the isoflurane group (P = 0.218) (Fig. 3). The median [IQR] CS 15-F2t-isoprostane level at reperfusion increased to 77.7 [56.3-124.4] pg.mL−1 in the propofol group (P = 0.004) vs 62.7 [42.46-104.4] pg.mL−1 in the isoflurane group (P = 0.084). Participants receiving propofol experienced a greater mean (SD) increase from baseline in CS 15-F2t-isoprostane levels compared with those receiving isoflurane group (26.9 (10.9) pg.mL−1 vs 12.1 (10.4) pg.mL−1, respectively; mean difference, 14.8; 95% CI, 11.0 to 18.6; P < 0.001).
Low cardiac output syndrome occurred in 49/125 (39.2%) participants, attributable to stunning (CS cTnI < 0.5 µg.L−1) in 24/49 (49%) episodes or intraoperative myocardial damage (CS cTnI > 0.5 µg.L−1) in 25/49 (51%) episodes.30,31 Compared with the isoflurane group, the incidence of LCOS was 1) lower overall in the propofol group (20.9% propofol vs 57.1% isoflurane; RR, 0.37; 95% CI, 0.22 to 0.62; P < 0.001); 2) similar between groups for participants without diabetes (26.1% propofol vs 38.4% isoflurane; RR, 0.68; 95% CI, 0.29 to 1.57; P = 0.382); and 3) decreased in the DM2 propofol subgroup (17.9% DM2 propofol vs 70.3% isoflurane; RR, 0.26; 95% CI, 0.13 to 0.52; P < 0.001; interaction P value = 0.08) (Table 2). Median [IQR] duration of inotropic support was 3.6 [2.0-6.9] hr in the propofol group vs 11.2 [4.7-18.0] hr in the isoflurane group (difference in the medians, 7.6 hr; 95% CI, 1.8 to 13.5; P = 0.004). Intra-aortic balloon pump counterpulsation was not required. Major adverse cardiac events (MACE) occurred in three participants in the propofol group vs 17 participants in the isoflurane group (RR, 0.26; 95% CI, 0.09 to 0.77; P = 0.001). Heart failure events decreased with propofol compared with isoflurane [0/62 (0%) vs 14/63 (22.2%), respectively; RR, 0.34; 95% CI, 0.19 to 0.59; P < 0.001] (Table 3).
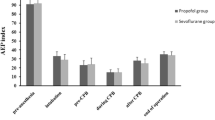
Median [IQR] cTnI values were similar at baseline, i.e., pre-CPB, but increased similarly in both groups at reperfusion, 12 hr, and 24 hr (P < 0.001) (Fig. 4). Sixteen participants had CS cTnI > 0.94 µg·L−1 at reperfusion (three participants in the propofol group vs 13 in the isoflurane group, P = 0.014), and nine participants had systemic cTnI ≥ 6.93 µg·L−1 in 24 hr (none in the propofol group vs nine in the isoflurane group, P = 0.003).31,32
Perioperative troponin I profiles. The squares denote individual measurements in the propofol group; the dots denote individual measurements in the isoflurane group; hashed lines denote coronary sinus or postoperative prognostic cTnI thresholds for infarct-related events. cTnI = cardiac troponin I; IQR = interquartile range; Pre-CPB = before cardiopulmonary bypass; repn = reperfusion after release of aortic cross clamp
The mean (SD) relative protein expression of myocardial Bcl-2 at reperfusion was 1.43 (0.94) in the propofol group vs 0.77 (0.52) in the isoflurane group (mean difference, 0.65; 95% CI, 0.29 to 1.01; P = 0.005) (Fig. 5).
Myocardial Bcl-2 protein expression at reperfusion. The dots denote the individual measurements determined by Western blotting. The long horizontal lines and the vertical lines with cross bars denote the means and standard deviations, respectively, in each group. Values greater than one denote upregulation or gain. Values less than one denote downregulation or loss. Bcl-2 = myocardial B-cell lymphoma 2
The mean (SD) propofol concentration was 5.74 (2.50) μg.mL−1 in systemic blood and 2.04 (1.14) μg.mL−1 in blood microplegia.
Discussion
In this study, our results did not confirm our hypothesis that propofol would decrease 15-F2t-isoprostane release. We showed that, compared with isoflurane, participants receiving propofol had higher CS levels of this marker of oxidative stress at reperfusion following CPB. Interestingly, the incidence, duration, and risk of LCOS were decreased in patients with DM2. The incidence of MACE, primarily heart failure events, was lowest in patients with DM2 treated with propofol cardioprotection. This may be attributable in part to a decreased incidence of severe postoperative cardiac injury. B-cell lymphoma 2, a mitochondrial protectant, increased in myocardium at reperfusion in participants receiving propofol. Propofol could mediate a pro-oxidant mechanism of cardioprotection.
Oxidative stress is a major factor in the pathophysiology of myocardial IRI (Fig. 6).12,13,21 During ischemia, the mitochondrial electron transport chain (ETC) is damaged. B-cell lymphoma 2, a mitochondrial protein, is depleted.21 During reperfusion, reactive oxygen species (ROS) generated by the damaged ETC induce opening of the mitochondrial permeability transition pore (mPTP), a non-specific channel of the inner mitochondrial membrane. Permeation is enhanced by translocation of the proapoptotic effector protein, BAX, from cytosol to mitochondria. Oxidative phosphorylation becomes uncoupled, increasing ROS production and release of cell death activators that induce mitochondrial rupture.12 While the effects of ROS are largely compartmentalized within cells, they trigger generation of stable diffusible lipid peroxidation products like 15-F2t-isoprostane, a coronary artery vasoconstrictor implicated in the development of postoperative ventricular dysfunction.19,26
Proposed mechanism of injury from ischemia-reperfusion. Loss of Bcl-2 secondary to ischemic damage to the electron transport chain predisposes activation of the mitochondrial death pathway, alteration of cardiac energetics, and development of cellular acidosis resulting in cardiac injury and dysfunction. Bcl-2 = myocardial B-cell lymphoma 2; LCOS = low cardiac output syndrome; MACE = major adverse cardiac events
We attribute our findings to patient characteristics, surgical conditions, and study interventions that differ from prior studies. We studied higher risk patients undergoing normothermic CPB and blood cardioplegic arrest. We included patients prescribed oral hypoglycemic agents or statins known to inhibit pre/postconditioning. Our study intervention was derived from prior in vitro and in vivo studies of the concentration and time-dependent effects of propofol which define a therapeutic window that we correlate with cyto- and cardioprotection (4.2-8.4 µg·mL−1).16,17,23 These concentrations were achieved in the systemic circulation at reperfusion in 87% of participants where aortic cross-clamp intervals exceeded 60 min. Our technique enriches blood cardioplegia. Propofol 2 µg·mL−1 was delivered in 300 mL·min−1 boluses every 15-20 min during global ischemia. Compared with isoflurane, this regimen appears to decrease transient LCOS episodes and acute heart failure events secondary to myocardial stunning or injury.
In contrast, myocardial injury increased in low-risk patients following propofol anesthesia for ACBP surgery conducted with hypothermic CPB and cold crystalloid or blood cardiopreservation.14,33 Membrane fluidity decreases in the presence of propofol at 25°C.34 This could disrupt pharmacologic recruitment of cardioprotective signal transduction.35,36 Detoxification of ROS by phenolic antioxidants involves conversion to peroxide and a phenoxyl radical that stimulates DNA fragmentation.12 Furthermore, propofol induced transcriptional changes in fatty acid metabolism and DNA damage signalling, correlating with release of N- terminal pro-brain natriuretic peptide (NT-proBNP), a biomarker of cardiac dysfunction.37 Propofol concentrations were not measured and were likely suboptimal. Interestingly, a recent randomized multicentre trial concluded that inhalational anesthesia was not superior to total intravenous anesthesia in patients undergoing high-risk cardiac surgery.8 The authors suggested that the benefits of inhalational anesthesia may be limited to low-risk surgical settings.
Similar 15-F2t-isoprostane results were reported following off-pump coronary artery bypass graft surgery.38 Although counterintuitive, there are two potential explanations. First, inhalational anesthesia may protect by suppressing burst of free radicals at reperfusion, inhibiting the translocation of BAX from cytosol to mitochondria.39Nevertheless, Bcl-2 levels are decreased in the diabetic or aged heart and subject to ischemic degradation during surgery.21,28 If the buffering capacity of Bcl-2 is exhausted, BAX would initiate mitochondrial permeability transition, and cell injury would ensue.21 Second, instead of scavenging ROS, propofol may initiate ROS-mediated signalling events whereby cardiomyocytes opt for cell survival.20,21 Sublethal oxidative stress could trigger adaptive stress resistance via the upregulation of proteins like Bcl-2 to control mPTP opening.40 Unlike inhalational anesthesia, the mechanism may involve transcription factor STAT3, which doesn’t require a second messenger system for signal transduction.41 Release of ROS would subsequently be attenuated, and 15-F2t-isoprostane generation would decline. This protective response, termed mitochondrial hormesis (i.e., mitohormesis), could prevent injury and remodelling of the ischemic-reperfused heart.42,43
In mitohormesis, mitochondria generate ROS as part of a cellular adaptation involving downregulation of the cellular respiration.44 This preserves Bcl-2 and inhibits the mPTP.45 B-cell lymphoma 2 prevents the decline in pH and cardiac adenosine triphosphate (ATP) stores during ischemia.46 Metabolic regulation shifts from glucose to fatty acid oxidation to restore cellular homeostasis.44 Concern has been raised over the potential negative impact of propofol-mediated insulin resistance and fatty acid oxidation on the heart.47Nevertheless, fatty acid oxidation reverses an energy deficit and improves function of the post-ischemic insulin-resistant heart.48 This could explain discrepancies in the incidence of LCOS episodes and events of heart failure seen in our study. Given our results, the areas of ongoing research by our laboratory include the molecular mechanism, functional significance, and therapeutic impact of propofol cardioprotection in patients with type 2 diabetes.
Our findings should be considered hypothesis-generating, not confirmatory, and should be interpreted with caution. Our phase 2 study design was based on the pharmacology of propofol. Our approach to CPB and cardioplegia protection may not reflect the practices of other institutions. The increase in 15-F2t-isoprostane and decrease in LCOS in response to propofol may be incidental and not reflective of a cardioprotective mechanism. Cardiac events in the isoflurane group could have arisen by chance or could be secondary to other factors, including microembolization, inadequate revascularization, or other mediators of cardiac injury.1,6 In patients without diabetes, we cannot differentiate between the nonexistence of effect and the lack of power to detect it. Our clinical observations from the diabetes subgroup require further validation. Also, we cannot exclude a synergistic mechanism of protection involving propofol and insulin by which to explain our results.49,50 Finally, our analysis of cTnI was exploratory. We did not incorporate sophisticated measures of cardiac injury and areas at risk in this study.
In summary, compared with isoflurane, continuous, systemic, and intermittent delivery of propofol-enriched microplegia during ischemia-reperfusion elicited a prooxidant response associated with decreased LCOS episodes and heart failure events following primary ACBP surgery. Propofol may be a preemptive intraoperative cardioprotectant for patients with DM2 under conditions of normothermic bypass and blood cardioplegic arrest. Further mechanistic studies and larger phase 3 clinical trials are needed to acquire better clarification regarding this possible effect.
References
Haunsenloy DJ, Boston-Griffiths, Yellon DM. Cardioprotection during cardiac surgery. Cardiovasc Res 2012; 94: 253-65.
Verma S, Farkouh ME, Yanagawa B, et al. Comparison of coronary artery bypass surgery and percutaneous coronary intervention in patients with diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol 2013; 1: 317-28.
Algarni KD, Maganti M, Yau TM. Predictors of low cardiac output syndrome after isolated coronary bypass surgery: trends over 20 years. Ann Thorac Surg 2011; 92: 1678-85.
Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986; 74: 1124-36.
De Hert SG. Is anaesthetic cardioprotection clinically relevant? Another futile search for a magic bullet? Eur J Anaesthesiol 2011; 28: 616-7.
Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet 2013; 381: 166-75.
Zaugg M, Lucchinetti E, Behmanesh S, Clanachan AS. Anesthetic cardioprotection in clinical practice from proof-of-concept to clinical applications. Curr Pharm Des 2014; 20: 5706-26.
Landoni G, Guarracino F, Cariello C, et al. Volatile compared to total intravenous anesthesia in patients undergoing high-risk cardiac surgery: a randomized multicentre study. Br J Anaesth 2014; 113: 955-63.
Wojtovich AP, Nadtochiy SM, Brookes PS, Nehrke K. Ischemic preconditioning: the role of mitochondria and aging. Exp Gerontol 2012; 47: 1-7.
Fullmer TM, Pei S, Zhu Y, et al. Insulin suppresses ischemic-preconditioning mediated cardioprotection through Akt-dependent mechanisms. J Mol Cell Cardiol 2013; 64: 20-9.
Anderson EJ, Rodriguez E, Anderson CA, Thayne K, Chitwood WR, Kypson AP. Increased propensity for cell death in diabetic human heart is mediated by mitochondrial-dependent pathways. Am J Physiol Heart Circ Physiol 2011; 300: H118-24.
Raedschelders K, Ansley DM, Chen DD. The cellular and molecular origins of reactive species generation during myocardial ischemia and reperfusion. Pharmacol Ther 2012; 133: 230-55.
Ansley DM, Wang B. Oxidative stress and myocardial injury in the diabetic heart. J Pathol 2013; 229: 232-41.
Yu CH, Beattie WS. The effects of volatile anesthetics on cardiac ischemic complications and mortality in CABG: a meta-analysis. Can J Anesth 2006; 53: 906-18.
Pagel PS. Myocardial protection by volatile anesthetics in patients undergoing cardiac surgery: a critical review of the laboratory and clinical evidence. J Cardiothorac Vasc Anesth 2013; 27: 972-82.
Runzer DT, Ansley DM, Godin DV, Chambers GK. Tissue antioxidant capacity during anesthesia: propofol enhances in vivo red cell and tissue antioxidant capacity in a rat model. Anesth Analg 2002; 94: 89-93.
Xia Z, Godin DV, Chang TK, Ansley DM. Dose-dependent protection of cardiac function by propofol during ischemia and early reperfusion in rats: effects on 15-F2t-isoprostane formation. Can J Physiol Pharmacol 2003; 81: 14-21.
Xia Z, Godin DV, Ansley DM. Propofol enhances ischemic tolerance of middle-aged rat hearts: effects on 15-F(2t)-isoprostane formation and tissue antioxidant capacity. Cardiovasc Res 2003; 59: 113-21.
Mobert J, Becker BF, Zahler S, Gerlach E. Hemodynamic effects of isoprostanes (8-iso-prostaglandin F2α and E2) in isolated guinea pig hearts. J Cardiovasc Pharmacol 1997; 29: 789-94.
Wang B, Shravah J, Luo H, Raedschelders K, Chen DD, Ansley DM. Propofol protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via Akt activation and Bcl-2 up-regulation. Biochem Biophys Res Commun 2009; 389: 105-11.
Szczepanek K, Chen Q, Larner AC, Lesnefsky EJ. Cytoprotection by the modulation of mitochondrial electron transport chain: the emerging role of mitochondrial STAT3. Mitochondrion 2012; 12: 180-9.
Ansley DM, Xia Z, Dhaliwal BS. The relationship between plasma 15-F2t-isoprostane concentration and early postoperative cardiac depression following warm heart surgery. J Thorac Cardiovasc Surg 2003; 126: 1222-3.
Raedschelders K, Hui Y, Laferlita B, et al. Target-achieved propofol concentration during on-pump cardiac surgery: a pilot dose-finding study. Can J Anesth 2009; 56: 658-66.
Ansley DM, Raedschelders K, Chen DD, Choi PT. Rationale, design and baseline characteristics of the PRO-TECT II study: PROpofol CardioproTECTion for type II diabetics: a randomized, controlled trial of high-dose propofol versus isoflurane preconditioning in patients undergoing on-pump coronary artery bypass graft surgery. Contemp Clin Trials 2009; 30: 380-5.
Sircar D, Subbaiah PV. Isoprostane measurement in plasma and urine by liquid chromatography-mass spectrometry with one-step sample preparation. Clin Chem 2007; 53: 251-8.
Xia Z, Kuo KH, Godin DV, Walker MJ, Tao MC, Ansley DM. 15-F2t-isoprostane exacerbates myocardial ischemia-reperfusion injury of isolated rat hearts. Am J Physiol Heart Circ Physiol 2005; 289: H1366-72.
Hui Y, Raedschelders K, Zhang H, Ansley DM, Chen DD. Quantitative analysis of propofol in whole blood using capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877: 703-9.
Wang B, Raedschelders K, Shravah J, et al. Differences in myocardial PTEN expression and Akt signaling in type 2 diabetic and non-diabetic patients undergoing coronary bypass surgery. Clin Endocrinol (Oxf) 2011; 74: 705-13.
Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015; DOI: 10.1016/j.jacc.2014.12.018.
Eigel P, van Ingen G, Wagenpfeil S. Predictive value of perioperative cardiac troponin I for adverse outcome in coronary artery bypass surgery. Eur J Cardiothorac Surg 2001; 20: 544-9.
Ornorati F, Cristodoro L, Caroleo S, et al. Troponin I and lactate from coronary sinus predict cardiac complications after myocardial revascularization. Ann Thorac Surg 2007; 83: 1016-23.
Muehlschlegel JD, Perry TE, Liu KY, et al. CABG Genomics Investigators. Troponin is superior to electrocardiogram and creatinine kinase MB for predicting clinically significant myocardial injury after coronary artery bypass grafting. Eur Heart J 2009; 30: 1574-83.
Julier K, da Silva R, Garcia C, et al. Preconditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: a double-blinded, placebo-controlled, multicentre study. Anesthesiology 2003; 98: 1315-27.
Bahri MA, Seret A, Hans P, Piette J, Deby-Dupont G, Hoebeke M. Does propofol alter membrane fluidity at clinically relevant concentrations? An ESR spin label study. Biophys Chem 2007; 129: 82-91.
Smul TM, Stumpner J, Blomeyer C, et al. Propofol inhibits desflurane-induced preconditioning in rabbits. J Cardiothorac Vasc Anesth 2011; 25: 276-81.
Kottenberg E, Thielmann M, Bergmann L, et al. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol – a clinical trial. Acta Anaesthesiol Scand 2012; 56: 30-8.
Lucchinetti E, Hofer C, Bestmann L, et al. Gene regulatory control of myocardial energy metabolism predicts postoperative cardiac function in patients undergoing off-pump coronary artery bypass graft surgery–inhalational versus intravenous anesthetics. Anesthesiology 2007; 106: 444-57.
Ballester M, Llorens J, Garcia de-la-Asuncion J, et al. Myocardial oxidative stress protection by sevoflurane vs. propofol: a randomized controlled study in patients undergoing off-pump coronary artery bypass graft surgery. Eur J Anaesthesiol 2011; 28: 874-81.
Zu L, Zheng X, Wang B, et al. Ischemic preconditioning attenuates mitochondrial localization of PTEN induced by ischemia-reperfusion. Am J Physiol Heart Circ Physiol 2011; 300: H2177-86.
Javadov SA, Lim KH, Kerr PM, Suleiman MS, Angelini GD, Halestrap AP. Protection of hearts from reperfusion injury by propofol is associated with inhibition of the mitochondrial permeability transition. Cardiovasc Res 2000; 45: 360-9.
Shravah J, Wang B, Pavlovic M, et al. Propofol mediates signal transducer and activator of transcription 3 activation and crosstalk with phosphoinositide 3-kinase/AKT. JAKSTAT 2014; 3: e29554.
Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med 2014; 20: 709-11.
Schwartz DR, Sack MN. Targeting the mitochondria to augment myocardial protection. Curr Opin Pharmacol 2008; 8: 160-5.
Ristow M, Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response 2014; 12: 288-341.
Chen Q, Lesnefsky EJ. Blockade of electron transport during ischemia preserves bcl-2 and inhibits opening of the mitochondrial permeability transition pore. FEBS Lett 2011; 585: 921-6.
Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res 2004; 95: 734-41.
Lou PH, Lucchinetti E, Zhang L, et al. Propofol (Diprivan®) and Intralipid® exacerbate insulin resistance in type-2 diabetic hearts by impairing GLUT 4 trafficking. Anesth Analg 2015; 120: 329-40.
Harmancey R, Vasquez HG, Guthrie PH, Taegtmeyer H. Decreased long-chain fatty acid oxidation impairs postischemic recovery of the insulin-resistant rat heart. FASEB J 2013; 27: 3966-78.
Chaudhuri A, Janicke D, Wilson M, et al. Effect of modified glucose-insulin-potassium on free fatty acids, matrix metalloproteinase, and myoglobin in ST-elevation myocardial infarction. Am J Cardiol 2007; 100: 1614-8.
Bell R, Kunuther SP, Hendry C, Bruce-Hickman D, Davidson S, Yellon DM. Matrix metalloproteinase inhibition protects CyPD knockout mice independently of RISK/mPTP signalling: a parallel pathway to protection. Bas Res Cardiol 2013; 108: 331-42.
Acknowledgements
We sincerely thank the patients and staff members (research coordinators, nurses, technicians, perfusionists, and fellows) of the Vancouver Coastal Health, Vancouver Acute Department of Anesthesiology and Perioperative Medicine, and Division of Cardiac Surgery for their assistance with this study. Drs. Guy Fradet, Peter Skarsgard, and Michael Janusz are cardiac surgeons who helped with study recruitment and collection of intraoperative specimens. Drs. Calvin Au and Paul Kapnoudhis are cardiac anesthesiologists who helped with conducting the study. We thank Yu Hui PhD for his help with structural analysis and Dr. Donald Griesdale for reviewing the manuscript prior to submission.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
David Ansley, Koen Raedschelders, David Chen, and Peter Choi designed the study. David Ansley, Peter Choi, Koen Raedschelders, and Baohua Wang coordinated the study. David Ansley, Baohua Wang, and Koen Raedschelders analyzed the data. Koen Raedschelders and David Chen conducted drug measurements and blood bioanalysis. Richard Cook helped with data acquisition. David Ansley was the principal investigator. Baohua Wang conducted tissue analysis. All authors contributed to critical revision of article content.
This article is accompanied by an editorial. Please see Can J Anesth 2016; 63: this issue.
This project was funded with peer-reviewed awards and grants from the International Anesthesia Research Society, Canadian Anesthesia Research Foundation, and the Canadian Institutes of Health Research (#82757). These organizations had no role in the study design, conduct, analysis or reporting. Koen Raedschelders was supported by a CIHR Canada Graduate Student Scholarship and CIHR Postdoctoral Fellowship.
Rights and permissions
About this article
Cite this article
Ansley, D.M., Raedschelders, K., Choi, P.T. et al. Propofol cardioprotection for on-pump aortocoronary bypass surgery in patients with type 2 diabetes mellitus (PRO-TECT II): a phase 2 randomized-controlled trial. Can J Anesth/J Can Anesth 63, 442–453 (2016). https://doi.org/10.1007/s12630-015-0580-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-015-0580-z