Abstract
Purpose
Several fluids are available for volume therapy to address hypovolemia. We focus on two hydroxyethyl starches (HES) available for volume expansion in Canada, HES 130/0.4 (Voluven®) and HES 260/0.45 (Pentaspan®). Although information is available regarding their pharmacokinetic and risk/benefit profiles, this paper examines their viscous properties.
Methods
Dynamic viscosities of HES 130/0.4 and HES 260/0.45 were measured through capillary viscometry at 21°C and 37°C. The viscosities of the solutions were then measured through a closed flow loop at room temperature across physiologically relevant flow rates that maintained a laminar flow regime.
Results
Measured dynamic viscosity through capillary viscometry for HES 130/0.4 and HES 260/0.45 was 2.76 centipoises (cP) and 7.62 cP, respectively, at 21°C decreasing to 1.74 cP and 4.25 cP, respectively, at 37°C. Pipe flow analysis found that HES 130/0.4 (expiry 02/13) and HES 260/0.45 (expiry 10/10) displayed marginal variation in viscosity suggesting Newtonian behaviour. However, a sample of HES 130/0.4 (expiry 10/10) displayed an appreciable increase in viscosity (13%) at higher flow rates suggesting shear thickening behaviour.
Conclusion
This study represents an innovative characterization of not only the viscosity of two commonly utilized HES solutions but also their viscous behaviour across physiologically relevant flow rates. The shear thickening behaviour of a sample of HES 130/0.40 (expiry 10/10) at high flow rates was not expected, and the effect this result may have on endothelial cell function is unknown.
Résumé
Objectif
Plusieurs liquides de remplissage sont disponibles pour le traitement d’une hypovolémie. Nous nous concentrerons sur deux produits d’expansion volumique à base d’amidon hydroxyéthylé (HES) disponibles au Canada: le HES 130/0.40 (Voluven®) et le HES 260/0.45 (Pentaspan®). Bien qu’une information soit disponible sur leurs profils pharmacocinétiques et risques/bénéfices, cet article s’intéresse à leurs propriétés visqueuses.
Méthodes
Les viscosités dynamiques du HES 130/0.4 et du HES 260/0.45 ont été mesurées par viscométrie capillaire à 21 °C et à 37 °C. La viscosité de ces solutions a ensuite été mesurée dans une boucle fermée à température ambiante avec des débits physiologiquement pertinents qui maintenaient un régime de flux laminaire.
Résultats
La viscosité dynamique mesurée par viscométrie capillaire pour le HES 130/0,4 et le HES 260/0,45 ont été, respectivement, de 2,76 centipoises (cP) et de 7,62 cP, à 21 °C, baissant respectivement à 1,74 cP et 4,25 cP à 37 °C. L’analyse du débit en conduite a montré que le HES 130/0,4 (date d’expiration: 02/13) et le HES 260/0,45 (date d’expiration: 10/10) présentaient une variation marginale de viscosité, suggérant un comportement newtonien. Cependant, un échantillon de HES 130/0,4 (date d’expiration: 10/10) a présenté une augmentation appréciable de la viscosité (13 %) pour des débits supérieurs, suggérant un comportement d’épaississement par cisaillement.
Conclusion
Cette étude présente une caractérisation innovante, non seulement de la viscosité de deux solutions fréquemment utilisées d’HES mais encore de leur comportement pour des débits physiologiquement pertinents. Le comportement d’épaississement par cisaillement d’un échantillon de HES 130/0.40 (date d’expiration: 10/10) à des débits élevés était inattendu et les conséquences potentielles de ce résultat sur la fonction des cellules endothéliales est inconnu.
Similar content being viewed by others
Hypovolemia often occurs in patients presenting with severe trauma or postoperatively after major surgery.1 During periods of low blood volume, the body attempts to compensate for this reduction through a redistribution of flow that may compromise perfusion rates to non-vital organs and the microvasculature.2,3 Effective management of blood volume replacement is thus paramount to ensure adequate peripheral tissue perfusion and oxygenation.2,4
Although the merits of effective volume replacement have been well established, there continues to be on-going debate regarding the ideal fluid for volume therapy.1,2,5-8
We focus here on two hydroxyethyl starch (HES) solutions available for volume expansion in Canada, 6% HES 130/0.4 (Voluven®, Fresenius Kabi, Bad Homburg, Germany) and 10% HES 260/0.45 (Pentaspan®, Bristol-Myers Squibb Canada, Montreal, QC, Canada). Although pharmacokinetic and risk/benefit profiles have been documented,9,10 this study examines simple mechanical properties, such as viscosity, that are of critical importance to endothelial cell function and the microvasculature.
Resuscitation with HES fluids reduces hematocrit, and depending on the viscosity of the infused fluid, it may decrease blood viscosity and lower wall shear stress (tangential stress experienced by endothelial cells caused by moving fluid) that can alter endothelial cell permeability, structural organization, and expression.7,11-14 The critical association between viscosity, shear stress, and endothelial function in the microcirculation and conduit arteries has been well established.12-20 Low and highly oscillatory shear stress patterns have been linked to intimal media thickening which promotes atherogenic development and decreased production of vasodilators fostering increased peripheral vascular resistance.12,14 Endothelial cells exposed to a range of physiological shear stress, typically between 0.5 and 2.0 Pascal (Pa), have been shown to elicit an atheroprotective phenotype.17-19 At elevated levels of shear, reorganization of endothelial cell orientation and shape has been observed, whereas, cells exposed to low and oscillatory patterns present with a rounded shape and fail to align with the direction of flow, increasing permeability and enhancing atherogenic expression.14,17,18 Maintenance of elevated shear through viscosity control enhances cytoskeleton remodelling and activates a signalling cascade, leading to the expression of vasodilators, nitric oxide, and prostacyclin that ensures preservation of adequate blood flow and a decrease in vascular resistance.12-14,16-18 Furthermore, flow-mediated dilation resulting from reactive hyperemia has been found to be proportional to levels of diastolic shear stress in the brachial artery whereupon diastolic flow transitions from stasis or reversal to continual forward flow.20
Given the critical association of viscosity with microvascular and endothelial cell function, this present study was undertaken to characterize the viscosity of HES 130/0.4 and HES 260/0.45 through capillary viscometry and pipe flow analysis over a range of shear rates commensurate with those of the vasculature. In light of recent developments that have called into question the efficacy of HES fluids, our work presented here allows for an appreciation of the physico-chemical properties of these fluids, which is critical to understand their effects on infusion.
Methods
The dynamic viscosities of 6% HES 130/0.4 (mean molecular weight 130 kilodaltons [kDa], molar substitution 0.4, expiration dates 10/10, 02/13)6 and 10% HES 260/0.45 (mean molecular weight 264 kDa, molar substitution 0.45, expiration date 10/10)21 were evaluated at room and body temperature (37°C) using a Cannon-Fenske routine calibrated CFRC (9721-B50) series size-50 capillary viscometer (Cannon Instrument Company, College Park, PA, USA). Measurements at 37°C were acquired in a temperature-controlled incubator. Samples of HES were stored at room temperature as per manufacturer specifications, and all measurements were acquired prior to date of expiration. Three measurements were acquired at each temperature for each HES. The final dynamic viscosity represented the average of the three measurements. The dynamic viscosities of Newtonian control fluids (fluid whereupon viscosity remains constant at all rates of shear) of deionized water and two HES viscosity-matching glycerol-water mixtures (30:70 glycerol-water, 50:50 glycerol-water) were also measured at room temperature.
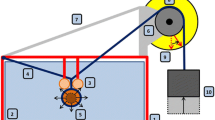
A closed flow loop was constructed to observe the viscous behaviours of HES 130/0.4 and HES 260/0.45 across a range of flow rates (Fig. 1). Fluid contained in an open reservoir was driven through the loop by an ISMATEC Reglo-Z Digital gear pump (Cole Parmer, Montreal, QC, Canada) using a Micropump pump head (Cole Parmer) with a flow range of 16 mL·min−1 to 4.5 L·min−1 at room temperature (21°C). A Validyne model P305D differential pressure transducer (Northridge, CA, USA) was calibrated for each working fluid prior to the measurement calculating pressure drop between two locations spaced 167 cm apart on a 183 cm long acrylic tube. The diameter of the tube was 0.635 cm, representative of the brachial artery. Despite the elastic component of arteries, a rigid tube was an appropriate representation of the brachial artery given its minimal relative distention of 1.5 (0.8)% and peak bluntness factor of 2.1 (value of 2.0 represents fully developed parabolic profile) that have allowed for close approximation in shear stress measurement using both the Poiseuille and Womersley equations that assume fully developed flow in rigid tubes.22,23
Initial maximum flow was to be restricted to a Reynolds Number (Re = inertial fluid forces / viscous fluid forces) of 1500 to negate potential transitional effects in the circulating fluid (transition to turbulence often commencing at Re ~ 2000). It was found, however, that Re could be increased safely to 1635 before transitional disturbances appeared. The flow range of the pump head restricted the minimum flow to 240 mL·min−1. These restrictions corresponded to flows of 240 mL·min−1 to 1.35 L·min−1 for HES 130/0.4 and flows of 660 mL·min−1 to 3.72 L·min−1 for HES 260/0.45. These flow rates equated to mean velocities of 12.6 cm·sec−1 to 71.1 cm·sec−1 for HES 130/0.4 and mean velocities of 34.7 cm·sec−1 to 196.1 cm·sec−1 for HES 260/0.45, with the higher velocities being physiologically appropriate.19,24-26 Deionized water and the two glycerol-water mixtures were also pumped through the flow loop. The flow rate of deionized water was restricted to 450 mL·min−1 to maintain laminar flow. An inlet length of 73 cm from the pump to the first pressure tap ensured fully developed flow.
Pressure drop measurements were acquired at 100 Hz using custom designed software in NI LabVIEW 2009 Version 9.0.1 (National Instruments, Austin, TX, USA, 2009). Using appropriate pressure calibrations, pressure in Pa was calculated. Maintenance of a laminar flow regime allowed viscosity at our acquired flow rates to be calculated through Poiseuille’s equation (1):
μ: viscosity
R: radius of tube
ΔP: pressure drop
Q: flow
L: length between pressure taps
Shear rates for laminar flow were calculated using equation (2), which allowed for the derivation of shear stress using the calculated viscosities from the above equation (3). The reported viscosity and shear stress values for HES and matching glycerol-water mixtures represented the average of four independent pipe flow measurements.
γ: shear rate
Vavg: mean velocity
D: diameter of tube
τ: shear stress
γ: shear rate
μ: viscosity
Results
Dynamic viscosities for all measured fluids are shown in the Table. In Fig. 2, plots of shear stress vs shear rate are displayed for the five fluids investigated. Calculated viscosities from pipe flow measurements assuming a Newtonian fit of shear stress and shear rate were HES 130/0.4 (expiry 10/10) 2.74 (0.13) cP; HES 130/0.4 (expiry 02/13) 2.58 (0.05) cP; HES 260/0.45 6.97 (0.06) cP; 50:50 glycerol-water mixture 7.09 (0.08) cP; 30:70 glycerol-water mixture 2.38 (0.04) cP; and deionized water 0.99 (0.01) cP (Fig. 2a, b). The R2 values of the linear Newtonian fit ranged from 0.9986 for water to 0.9991 for HES 130/0.4 (Microsoft Excel 2007, Microsoft, Redmond, WA, USA, 2007).
The viscosity of deionized water measured across the range of flow rates varied < 2% from its nominal expected viscosity of 1.0 cP, while both glycerol-water mixtures varied < 2% from capillary viscometer measurements (Fig. 3a). The HES 130/0.4 sample (expiry 10/10) displayed Newtonian behaviour from mean velocities of 12.6 cm·sec−1 to 47.4 cm·sec−1 (Fig. 3b). An appreciable increase in viscosity was found at mean velocities > 47.4 cm·sec−1, increasing from 2.66 cP at 47.4 cm·sec−1 to 3.04 cP at 71.0 cm·sec−1 (13% increase) (Fig. 3b). The viscosity of HES 130/0.4 (expiry 02/13) varied from 2.67 cP to 2.53 cP (5.1%) from mean velocities of 12.6 cm·sec−1 to 22.1 cm·sec−1, respectively (Fig. 3b). From mean velocities of 31.6 cm·sec−1 to 71.0 cm·sec−1, viscosity varied from 2.55 cP to 2.63 cP (2.9%), respectively. HES 260/0.45 displayed Newtonian behaviour across the range of flow rates measured with viscosity ranging from 6.91 cP to 7.05 cP (2.0%) (Fig. 3a).
Pipe flow measured viscosity calculated by Poiseuille’s equation across a range of flow rates. (A) Hydroxyethyl starch (HES) 260/0.45 (expiry 10/10), glycerol-water mixture (50:50), HES 130/0.4 (expiry 10/10), HES 130/0.4 (expiry 02/13), glycerol-water mixture (30:70), and deionized water. (B) HES 130/0.4 (expiry 10/10) displaying shear thickening behaviour at high flow rates, HES 130/0.4 (expiry 02/13), and glycerol-water mixture (30:70)
Discussion
The focus of this study was on the viscous characterization of the two HES solutions available for volume expansion in Canada, HES 130/0.4 and HES 260/0.45. Hydroxyethyl starch products represent an attractive option to crystalloids or albumin due to improved plasma volume expansion and decreased costs. Concentrations of 6% HES have been found to induce an initial plasma volume expansion upwards of 20%, whereas the volume expansion effects of concentrations of 10% HES are assumed to be 1.45 times the infused volume.6,27 This provides volume ratios that are two to six times greater than those achieved with crystalloid solutions.27 Since pharmacokinetics and risk-benefit profiles of these HES solutions are extensively available, the aim of our study was to analyze the viscosity and viscous behaviour of the solutions. Our findings represent not only the characterization of the viscosity of HES 130/0.4 and HES 260/0.45, but also their viscous behaviour when subjected to pipe flow analysis simulating an arterial flow loop.
Results of capillary viscometry at 37°C showed that the viscosity of HES 130/0.4 (1.74 cP) was less than the viscosity of whole blood (3.5 cP), while the viscosity of HES 260/0.45 (4.25 cP) showed a value greater than that of whole blood. Both fluids showed viscosity values greater than published values for 5% human serum albumin (0.9 cP) and 10% human serum albumin (1.5 cP) at 37°C.16,28 Based on the capillary viscometry measurements at 37°C alone, we can infer that the infusion of HES 130/0.4 will initially decrease whole blood viscosity while the addition of HES 260/0.45 will increase blood viscosity. The clinical studies that have addressed this issue did not show significant changes in plasma viscosity, but neither study was designed to address acute changes following large volume resuscitation.8,29
Assuming a Newtonian relationship between shear stress and shear rate, pipe flow viscosities for the glycerol/water mixtures and deionized water were found to be in close agreement with those found through capillary viscometry (< 3%). Any discrepancies between the two measurements can likely be attributed to possible dilution of the HES solutions during pipe flow analysis (system was flushed with water between runs), small deviations during the manual calibration procedure, and the large pressure differences between the nominal pressure involved in capillary viscometry and the high pressures associated with pipe flow. Compared with capillary measured values, pipe flow viscosities for HES 130/0.4 and HES 260/0.45 were 6-8% lower. Room temperature at the time of pipe flow measurements of the HES fluids was 22°C, while their capillary viscometer measurements were acquired at 21°C. Assuming a linear decrease in viscosity between 21°C and 37°C, viscosities of HES 130/0.4 and HES 260/0.45 would be 2.63 cP and 7.20 cP, respectively, at 22°C or within 3% of pipe flow measured values.
As expected, control solutions of water and glycerol/water dilutions displayed Newtonian viscous behaviour. Our initial sample of HES 130/0.4 (expiry 10/10) displayed Newtonian properties from a flow rate of 4 mL·sec−1 to 15 mL·sec−1, whereupon an appreciable increase in viscosity from 2.66 cP to 3.04 cP (13% increase) occurred from a flow rate of 15 mL·sec−1 to 22.5 mL·sec−1, respectively. This result suggests fluid properties representative of a dilatant solution that is characterized by an increase in viscosity with shear rate. This finding was not expected and represents a revelation of possible shear thickening at high flow rates. Given this intriguing viscosity pattern, a second sample of HES 130/0.4 (expiry 02/13) was selected which showed a decrease in viscosity from a flow rate of 4 mL·sec−1 to 7 mL·sec−1. Viscosity then showed Newtonian behaviour with a variation in flow rate from < 3% up to a flow rate of 22.5 mL·sec−1. Based on this behaviour, we can likely conclude that this sample displays Newtonian viscous behaviour with nominal fluctuations across the range of measured flow rates. The only difference of note between the two HES 130/0.4 samples was the date of expiration (two and a half year difference).
As mentioned by Fall et al.,30 the mechanism under which shear thickening occurs has not yet been fully understood. However, it has been suggested that thickening in colloidal solutions may be the result of hydrodynamic cluster formation where viscosity is dependent on particle configuration or the aggregation of clusters creating a jammed system.30 Since the majority of complex fluids are shear thinning, comparison between Voluven® and other solutions is limited. One exception is cornstarch, and Fall et al.30 point out that its thickening properties are the result of dilatancy leading to a jammed system.
Clinical implications
The maintenance of functional capillary density (FCD) during prolonged hemorrhagic shock is critical to survival, and this is accomplished using high viscosity plasma expanders that ensure preservation of appropriate levels of shear.11,12,31 Salazar Vázquez et al.11 showed that the reduction in FCD, associated with hemodilution using a low viscosity fluid, is prevented when using a high viscosity expander. Salazar Vázquez et al.11 and Martini et al.12 observed that increasing plasma viscosity to 2.5 cP through the use of high viscosity expanders reduced peripheral vascular resistance, improved cardiac output, and allowed for a redistribution of pressure to the capillaries ensuring maintenance of red blood cell passage. Cytoskeletal changes in vitro have shown a rapid remodelling upon exposure to sudden increases in shear stress. This activates an atheroprotective signalling cascade in endothelial cells leading to the release of nitric oxide and prostacyclin that prevent platelet activation.14 Malek et al.18 observed that endothelial cell proliferation increased 18-fold upon exposure to low shear stress for 48 hours. This led to alterations in endothelial cell orientation, increased monocyte attachment and migration across the endothelial cell wall, and enhanced surface expression of adhesion molecules.18
What is not currently known is whether HES 130/0.4 and/or HES 260/0.45 elicit appreciable alterations to blood and plasma viscosity upon infusion. To attempt to quantify potential alterations, Einstein’s equation for spheres in suspension can be used. This was investigated by Walker et al.32 who modelled predicted changes in blood and plasma viscosity across a range of hematocrit upon infusion of HES 130/0.4 and HES 260/0.45. At low hematocrits, infusion of HES 260/0.45 was predicted to increase both blood and plasma viscosity, whereas HES 130/0.4 was predicted to decrease blood viscosity while simultaneously increasing plasma viscosity.32 It must be pointed out, however, that this model did not account for the effect of acute phase reactants on viscosity or the effect of these fluids on red blood cell adhesiveness.32
Given the differences in viscosity of these fluids, variations in infusion rates can also be expected. Stoneham33 compared the gravity-fed flow rates of 0.9% saline and Gelofusine, a colloid plasma substitute with a viscosity of 1.9 cP at 37°C, through a 14G cannula at room temperature.Footnote 1 A significantly lower flow rate was found for the more viscous Gelofusine fluid.33 The flow rate was also lower for Gelofusine when pressurized to 300 mmHg; however, the difference was clinically insignificant.33 It should be pointed out that the viscosity of Gelofusine is appreciably lower than HES 260/0.45, although it is comparable with HES 130/0.4 at 37°C (Table). Given the importance of rapid fluid replacement in hypovolemic patients, reduction in flow rate can be minimized by warming the HES to 37°C and by using pressure bags and large bore cannulas.33
Limitations
In this instance, we cannot conclude for certain the precise mechanism causing the shear thickening observed. Although manual calibrations of the pressure transducers were completed between trials, we find it difficult to believe this would be the sole reason for the discrepancies in viscosity patterns between these two samples. We suggest that the dilatant behaviour observed is similar to that of cornstarch whereupon friction between polymers is greatly increased at high shear rates. This finding could also be the result of an isolated difference in fluid properties between these particular samples, although we find this explanation to have little merit given that we observed these behaviours in two arbitrarily selected samples. Whether significant alterations in whole blood viscosity and associated shear stress would result upon infusion of a dilatant sample is the subject of future work.
We must also point out that testing was limited to three samples. Initially, one sample of each fluid was tested; however, a peculiar result was found with our initial sample of HES 130/0.4, prompting us to test using a second sample which happened to be a fresher batch. Sampling was limited initially to a single batch from each colloid, as one would expect marginal differences in intra-colloid viscosity. However, future investigations could include capillary viscosity measurements of multiple batches to confirm that differences in intra-colloid viscosity do in fact remain trivial.
Furthermore, we cannot say for certain if the HES solutions are undergoing changes to their viscosity as a function of storage time as it was simply our goal to characterize the viscous behaviour of these fluids in quasi-static conditions and under physiologically relevant flow rates. Although the issue of storage time and viscosity changes have been examined in the food and beverage industry,34,35 this has not been addressed in relation to HES fluids. Given the fortuitous result concerning HES 130/0.4, it is possible that storage life may affect the viscous behaviour of these fluids, and this has presented us with the opportunity to conduct a long-term study to examine viscous changes in multiple batches from date of receipt to expiration date.
In summary, this study represents characterization of the viscous properties of two HES solutions available for volume expansion in Canada, i.e., HES 130/0.4 and HES 260/0.45. The finding of non-Newtonian viscous behaviour in a sample of HES 130/0.4 with an expiration date of 10/10 was not expected, and this result has presented an opportunity to undertake further studies to determine the mechanism involved. We plan not only to examine the viscous behaviours of these solutions in pipe flow at body temperature (37°C), but also to undertake a long-term study examining the viscous properties of these solutions from date of receipt from distributor to their expiration date, including measuring viscosity at fixed monthly intervals. If viscosity profiles are found to change in response to time until expiration, a detailed examination into the chemical mechanism behind this would be warranted. This finding would be of significant interest to health care centres with a limited turnover of in-house volume expanders.
Notes
B Braun Medical. Gelofusine. Iso-oncotic gelatin solution for intravenous volume substitution. B Braun Melsungen AG, Melsungen, Germany, 2002.
References
Verheij J, van Lingen A, Beishuizen A, et al. Cardiac response is greater for colloid than saline fluid loading after cardiac or vascular surgery. Intensive Care Med 2006; 32: 1030-8.
Vincent JL, Gerlach H. Fluid resuscitation in severe sepsis and septic shock: an evidence-based review. Crit Care Med 2004; 32: S451-4.
Dubniks M, Persson J, Grande PO. Plasma volume expansion of 5% albumin, 4% gelatin, 6% HES 130/0.4, and normal saline under increased microvascular permeability in the rat. Intensive Care Med 2007; 33: 293-9.
Haljamae H, Dahlqvist M, Walentin F. 3 Artificial colloids in clinical practice: pros and cons. Baillières Clin Anaesthesiol 1997; 11: 49-79.
Traylor RJ, Pearl RG. Crystalloid versus colloid versus colloid: all colloids are not created equal. Anesth Analg 1996; 83: 209-12.
Westphal M, James MF, Kozek-Langenecker S, Stocker R, Guidet B, Van Aken H. Hydroxyethyl starches different products – different effects. Anesthesiology 2009; 111: 187-202.
Villela NR, Salazar Vazquez BY, Intaglietta M. Microcirculatory effects of intravenous fluids in critical illness: plasma expansion beyond crystalloids and colloids. Curr Opin Anaesthesiol 2009; 22: 163-7.
Woessner R, Grauer MT, Dieterich HJ, et al. Influence of a long-term, high-dose volume therapy with 6% hydroxyethyl starch 130/0.4 or crystalloid solution on hemodynamics, rheology and hemostasis in patients with acute ischemic stroke. Results of a randomized, placebo-controlled, double-blind study. Pathophysiol Haemost Thromb 2003; 33: 121-6.
Jungheinrich C, Neff TA. Pharmokinetics of hydroxyethyl starch. Clin Pharmacokinet 2005; 44: 681-99.
Treib J, Baron JF, Grauer MT, Strauss RG. An international view of hydroxyethyl starches. Intensive Care Med 1999; 25: 258-68.
Salazar Vazquez BY, Martini J, Chavez Negrete A, Cabrales P, Tsai AG, Intaglietta M. Microvascular benefits of increasing plasma viscosity and maintaining blood viscosity: counterintuitive experimental findings. Biorheology 2009; 46: 167-79.
Martini J, Cabrales P, Tsai AG, Intaglietta M. Mechanotransduction and the homeostatic significance of maintaining blood viscosity in hypotension, hypertension and haemorrhage. J Intern Med 2006; 259: 364-72.
Topper JN, Gimbrone MA Jr. Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today 1999; 5: 40-6.
Shaaban AM, Duerinckx AJ. Wall shear stress and early atherosclerosis: a review. AJR Am J Roentgenol 2000; 174: 1657-66.
Cabrales P, Tsai AG, Intaglietta M. Hyperosmotic-hyperoncotic versus hyperosmotic-hyperviscous: small volume resuscitation in hemorrhagic shock. Shock 2004; 22: 431-7.
Salazar Vazquez BY, Cabrales P, Intaglietta M. The beneficial effects of increasing blood viscosity. In: Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine 2008. : Springer; 2008. p. 691-700.
Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest 2005; 85: 9-23.
Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999; 282: 2035-42.
Reneman RS, Arts T, Hoeks AP. Wall shear stress – an important determinant of endothelial cell function and structure–in the arterial system in vivo. Discrepancies with theory. J Vasc Res 2006; 43: 251-69.
Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow-mediated dilation. The Framingham heart study. Hypertension 2004; 44: 134-9.
London MJ, Ho JS, Triedman JK, et al. A randomized clinical trial of 10% pentastarch (low molecular weight hydroxyethyl starch) versus 5% albumin for plasma volume expansion after cardiac operations. J Thorac Cardiovasc Surg 1989; 97: 785-97.
Dammers R, Stifft F, Tordoir JH, Hameleers JM, Hoeks AP, Kitslaar PJ. Shear stress depends on vascular territory: comparison between common carotid and brachial artery. J Appl Physiol 2003; 94: 485-9.
Simon AC, Levenson J, Flaud P. Pulsatile flow and oscillating wall shear stress in the brachial artery of normotensive and hypertensive subjects. Cardiovasc Res 1990; 24: 129-36.
Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343: 611-7.
Gisvold SE, Brubakk AO. Measurement of instantaneous blood-flow velocity in the human aorta using pulse Doppler ultrasound. Cardiovasc Res 1982; 16: 26-33.
Kilner PJ, Manzara CC, Mohiaddin RH, et al. Magnetic resonance jet velocity mapping in mitral and aortic valve stenosis. Circulation 1993; 87: 1239-48.
Degremont AC, Ismail M, Arthaud M, et al. Mechanisms of postoperative prolonged volume expansion with low molecular weight hydroxyethyl starch (HES 200/0.62, 6%). Intensive Care Med 1995; 21: 577-83.
Monkos K. On the hydrodynamics and temperature dependence of the solution conformation of human serum albumin from viscometry approach. Biochim Biophys Acta 2004; 1700: 27-34.
Kroemer H, Haass A, Müller K, et al. Haemodilution therapy in ischaemic stroke: plasma concentrations and plasma viscosity during long-term infusion of dextran 40 or hydroxyethyl starch 200/0.5. Eur J Clin Pharmacol 1987; 31: 705-10.
Fall A, Huang N, Bertrand F, Ovarlez G, Bonn D. Shear thickening of cornstarch suspensions as a reentrant jamming transition. Phys Rev Lett 2008; doi: 10.1103/PhysRevLett.100.018301.
Cabrales P, Tsai AG, Intaglietta M. Is resuscitation from hemorrhagic shock limited by blood oxygen-carrying capacity of blood viscosity? Shock 2007; 27: 380-9.
Walker AM, Lee K, Rinker KD, Shepherd RD, Dobson GM, Johnston CR. Effects of starch volume expanders on blood viscosity and vascular endothelial markers. The 2010 ASME International Mechanical Engineering Congress & Exposition. Available from URL : http://www.asmeconferences.org/Congress2010/TechnicalProgramOverview.cfm (accessed November 2011).
Stoneham MD. An evaluation of methods of increasing the flow rate of i.v. fluid administration. Br J Anaesth 1995; 75: 361-5.
Cano-Ruiz ME, Richter RL. Changes in physicochemical properties of retort-sterilized dairy beverages during storage. J Dairy Sci 1998; 81: 2116-23.
Severa L, Nedomova S, Buchar J. Influence of storing time and temperature on the viscosity of egg yolk. J Food Eng 2010; 96: 266-9.
Acknowledgement
The authors sincerely thank the Canadian Blood Services for providing samples of HES 130/0.4 and HES 260/0.45.
Funding provided by
National Sciences and Engineering Research Council of Canada (NSERC).
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walker, A.M., Lee, K., Dobson, G.M. et al. The viscous behaviour of HES 130/0.4 (Voluven®) and HES 260/0.45 (Pentaspan®). Can J Anesth/J Can Anesth 59, 288–294 (2012). https://doi.org/10.1007/s12630-011-9648-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-011-9648-6