Abstract
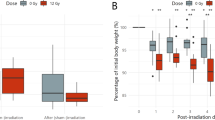
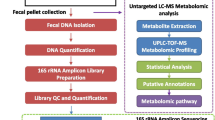
The purpose of this study was to investigate the role of Lactobacillus rhamnosus GG (LGG) probiotics in radiation enteritis using in vivo mice. A total of 40 mice were randomly assigned to four groups: control, probiotics, radiotherapy (RT), and RT + probiotics. For the group of probiotics, 0.2 mL of solution that contained 1.0 × 108 colony-forming units (CFU) of LGG was used and orally administered daily until sacrifice. For RT, a single dose of 14 Gy was administered using a 6 mega-voltage photon beam to the abdominopelvic area. Mice were sacrifice at day 4 (S1) and day 7 (S2) after RT. Their jejunum, colon, and stool were collected. A multiplex cytokine assay and 16 s ribosomal RNA amplicon sequencing were then performed. Regarding cytokine concentrations in tissues, pro-inflammatory cytokines, such as tumor necrosis factor-α, interleukin-6 and monocyte chemotactic protein-1, showed significantly decreased protein levels in colon tissues of the RT + probiotics group than in the RT alone group (all p < 0.05). As for comparing microbial abundance through alpha-diversity and beta-diversity, no significant differences were observed between the RT + probiotics and RT alone groups, except for an increase in alpha-diversity in the stool of the RT + probiotics group. Upon analysis of differential microbes based on treatment, the dominance of anti-inflammatory-related microbes, such as Porphyromonadaceae, Bacteroides acidifaciens, and Ruminococcus, was observed in the jejunum, colon, and stool of the RT + probiotics group. With regard to predicted metabolic pathway abundances, the pathways associated with anti-inflammatory processes, such as biosynthesis of pyrimidine nucleotides, peptidoglycans, tryptophan, adenosylcobalamin, and propionate, were differentially identified in the RT + probiotics group compared to the RT alone group. Protective effects of probiotics on radiation enteritis were potentially derived from dominant anti-inflammation-related microbes and metabolites.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are openly available in figshare at https://doi.org/10.6084/m9.figshare.21101686, and within the article and its supplementary materials. The 16s ribosomal RNA raw sequencing data can be found at the BioProject database (ID: PRJNA934953).
Abbreviations
- RT:
-
Radiotherapy
- CFU:
-
Colony-forming units
- LGG:
-
Lactobacillus rhamnosus GG
- TNF-α:
-
Tumor Necrosis Factor-α
- IL-6:
-
Interleukin-6
- MCP-1:
-
Monocyte Chemotactic Protein-1
- IL-12p40:
-
Interleukin-12p40
- IL-1α:
-
Interleukin-1α
- IL-1β:
-
Interleukin-1β
- IL-10:
-
Interleukin-10
- MPO:
-
Myeloperoxidase
- NMDS:
-
Non-metric multidimensional scaling
- LDA:
-
Linear discriminant analysis
- ANOVA:
-
One-way analysis of variance
- FDR:
-
False Discovery Rate
- SCFA:
-
Short-chain fatty acids
References
Andreyev HJ, Vlavianos P, Blake P, Dearnaley D, Norman AR, Tait D (2005) Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist? Int J Radiat Oncol Biol Phys 62(5):1464–1471
McGough C, Baldwin C, Frost G, Andreyev HJ (2004) Role of nutritional intervention in patients treated with radiotherapy for pelvic malignancy. Br J Cancer 90(12):2278–2287
Tharavichtikul E, Meungwong P, Chitapanarux T et al (2014) The association of rectal equivalent dose in 2 Gy fractions (EQD2) to late rectal toxicity in locally advanced cervical cancer patients who were evaluated by rectosigmoidoscopy in Faculty of Medicine, Chiang Mai University. Radiat Oncol J 32(2):57–62
Bismar MM, Sinicrope FA (2002) Radiation enteritis. Curr Gastroenterol Rep 4(5):361–365
O’Hara AM, Shanahan F (2006) The gut flora as a forgotten organ. EMBO Rep 7:688–693
Gerassy-Vainberg S, Blatt A, Danin-Poleg Y et al (2018) Radiation induces proinflammatory dysbiosis: transmission of inflammatory susceptibility by host cytokine induction. Gut 67:97–107
Zhao T-S, Xie L-W, Cai S et al (2021) Dysbiosis of Gut Microbiota Is Associated With the Progression of Radiation-Induced Intestinal Injury and Is Alleviated by Oral Compound Probiotics in Mouse Model. Front Cell Infect Microbiol 11:717636. https://doi.org/10.3389/fcimb.2021.717636
Ki Y, Kim W, Cho H, Ahn K, Choi Y, Kim D (2014) The effect of probiotics for preventing radiation-induced morphological changes in intestinal mucosa of rats. J Korean Med Sci 29(10):1372–1378
Delia P, Sansotta G, Donato V et al (2007) Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol 13(6):912–915
Demirer S, Aydintug S, Aslim B et al (2006) Effects of probiotics on radiation-induced intestinal injury in rats. Nutrition (Burbank, Los Angeles County, Calif) 22:179–186
Salminen E, Elomaa I, Minkkinen J, Vapaatalo H, Salminen S (1988) Preservation of intestinal integrity during radiotherapy using live Lactobacillus acidophilus cultures. Clin Radiol 39:435–437
Delia P, Sansotta G, Donato V et al (2002) Prophylaxis of diarrhoea in patients submitted to radiotherapeutic treatment on pelvic district: personal experience. Dig Liver Dis 34(Suppl 2):S84–S86
Ciorba MA et al (2012) Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 61(6):829–838
Guo H, Chou WC, Lai Y et al (2020) Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 370(6516):eaay9097
Ijssennagger N, van der Meer R, van Mil SWC (2016) Sulfide as a mucus barrier-breaker in inflammatory bowel disease? Trends Mol Med 22(3):190–199. https://doi.org/10.1016/j.molmed.2016.01.002
Morgan XC, Tickle TL, Sokol H et al (2012) Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 13(9):R79
Zackular JP, Baxter NT, Iverson KD et al (2013) The gut microbiome modulates colon tumorigenesis. mBio 4(6):e00692-13
Fardini Y, Chung P, Dumm R, Joshi N, Han YW (2010) Transmission of diverse oral bacteria to murine placenta: Evidence for the oral microbiome as a potential source of intrauterine infection. Infect Immun 78(4):1789–1796. https://doi.org/10.1128/IAI.01395-09
Willing BP, Dicksved J, Halfvarson J et al (2010) A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 139:1844–54.e1
Okesli A, Khosla C, Bassik MC (2017) Human pyrimidine nucleotide biosynthesis as a target for antiviral chemotherapy. Curr Opin Biotechnol 48:127–134. https://doi.org/10.1016/j.copbio.2017.03.010
Matsumoto S, Hara T, Nagaoka M et al (2009) A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis-associated cancer. Immunology 128:e170–e180
Sharon G, Garg N, Debelius J, Knight R, Dorrestein PC, Mazmanian SK (2014) Specialized metabolites from the microbiome in health and disease. Cell Metab 20(5):719–730. https://doi.org/10.1016/j.cmet.2014.10.016
Averina OV, Poluektova EU, Marsova MV, Danilenko VN (2021) Biomarkers and utility of the antioxidant potential of probiotic lactobacilli and bifidobacteria as representatives of the human gut microbiota. Biomedicines 9:1340
Schirmer M, Smeekens SP, Vlamakis H et al (2016) Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 167:1125–36.e8
Zheng C, Yu Z, Du C et al (2020) 2-Methylcitrate cycle: a well-regulated controller of Bacillus sporulation. Environ Microbiol 22(3):1125–1140. https://doi.org/10.1111/1462-2920.14901
Tong LC, Wang Y, Wang ZB et al (2016) Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front Pharmacol 15(7):253. https://doi.org/10.3389/fphar.2016.00253
Agus A, Richard D, Faïs T et al (2021) Propionate catabolism by CD-associated adherent-invasive E. coli counteracts its anti-inflammatory effect. Gut Microbes 13:1–18
LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P (2017) Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact 16:1–10
Wang K, Xu X, Maimaiti A et al (2021) Gut microbiota disorder caused by diterpenoids extracted from Euphorbia pekinensis aggravates intestinal mucosal damage. Pharmacol Res Perspect 9(5):e00765. https://doi.org/10.1002/prp2.765
Wang Z, Wang Q, Wang X et al (2019) Gut microbial dysbiosis is associated with development and progression of radiation enteritis during pelvic radiotherapy. J Cell Mol Med 23(5):3747–3756. https://doi.org/10.1111/jcmm.14289
Bai AP, Ouyang Q, Xiao XR, Li SF (2006) Probiotics modulate inflammatory cytokine secretion from inflamed mucosa in active ulcerative colitis. Int J Clin Pract 60(3):284–288. https://doi.org/10.1111/j.1368-5031.2006.00833.x
Ott SJ, Musfeldt M, Wenderoth DF et al (2004) Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 53:685–693
Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI (2014) Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med 6(220):220ra11. https://doi.org/10.1126/scitranslmed.3008051
Petersen C, Round JL (2014) Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol 16(7):1024–1033. https://doi.org/10.1111/cmi.12308
Holzapfel WH, Haberer P, Snel J, Schillinger U, Huis In’T Veld JHJ (1998) Overview of gut flora and probiotics. Int J Food Microbiol 41(2):85–101. https://doi.org/10.1016/s0168-1605(98)00044-0
Johnson LB, Riaz AA, Adawi D et al (2004) Radiation enteropathy and leucocyte-endothelial cell reactions in a refined small bowel model. BMC Surg 4:10
Kim YS, Kim J, Park S-J (2015) High-throughput 16S rRNA gene sequencing reveals alterations of mouse intestinal microbiota after radiotherapy. Anaerobe 33:1–7
Suzuki T, Yoshida S, Hara H (2008) Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr 100(2):297–305
Lomax AR, Calder PC (2009) Prebiotics, immune function, infection and inflammation: A review of the evidence. Br J Nutr 101(5):633–658. https://doi.org/10.1017/S0007114508055608
Göker M, Gronow S, Zeytun A et al (2011) Complete genome sequence of odoribacter splanchnicus type strain (1651/6 T). Stand Genomic Sci 4(2):200–209. https://doi.org/10.4056/sigs.1714269
Ze X, Duncan SH, Louis P, Flint HJ (2012) Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J 6:1535–1543
Weaver LT (1999) Helicobacter pylori in the faeces? QJM 92:361–364
Dolan B, Burkitt-Gray L, Shovelin S et al (2018) The use of stool specimens reveals Helicobacter pylori strain diversity in a cohort of adolescents and their family members in a developed country. Int J Med Microbiol 308(2):247–255. https://doi.org/10.1016/j.ijmm.2017.11.005
Hamilton-Miller JMT (2003) The role of probiotics in the treatment and prevention of Helicobacter pylori infection. Int J Antimicrob Agents 22(4):360–366. https://doi.org/10.1016/s0924-8579(03)00153-5
Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A (2019) Mechanisms of action of probiotics. Adv Nutr 10(suppl_1):S49–S66. https://doi.org/10.1093/advances/nmy063
Heintz-Buschart A, Wilmes P (2018) Human gut microbiome: function matters. Trends Microbiol 26(7):563–574. https://doi.org/10.1016/j.tim.2017.11.002
Jang B-S, Chang JH, Chie EK et al (2020) Gut microbiome composition is associated with a pathologic response after preoperative chemoradiation in patients with rectal cancer. Int J Radiat Oncol Biol Phys 107(4):736–746. https://doi.org/10.1016/j.ijrobp.2020.04.015
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant Number: 2019R1A2C1002071), the New Faculty Startup Fund, 3020200090 from SNUH Research Fund, and funded by the Seoul Metropolitan Government-Seoul National University Boramae Medical Center (Grant Number 03-2017-19).
Author information
Authors and Affiliations
Contributions
SL and BJ were involved in the conception design, analysis and interpretation of the data, and drafting of the manuscript. YN and SL collected and analyzed the data and assisted in manuscript drafting. SH collected and analyzed the data. JC and HK developed the conception design, analyzed and interpreted the data, and revised the manuscript critically for intellectual content.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The Institutional animal care and use committee of Seoul National University Hospital approved the experimental protocol (IACUC NO. 18–0096).
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, S.U., Jang, BS., Na, Y.R. et al. Effect of Lactobacillus Rhamnosus GG for Regulation of Inflammatory Response in Radiation-Induced Enteritis. Probiotics & Antimicro. Prot. 16, 636–648 (2024). https://doi.org/10.1007/s12602-023-10071-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-023-10071-9