Abstract
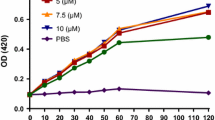
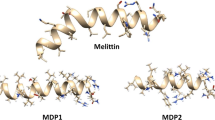
The low stability and nonspecific toxicity are the main limiting factors for the clinical applications of melittin (MLT). This study aimed to design and synthesize new analogs of MLT to increase stability, reduce toxicity, and retain their antimicrobial properties against bacterial pathogens. At first, peptide analogs were designed computationally by inducing single mutations in MLT peptides and evaluating their physicochemical properties. The stability of the analogs with the highest scores was determined by Gromacs software. In vitro assays were performed to examine the antimicrobial activity and toxicity of the selected analogs. Two peptide analogs, M1 and M2, were selected based on the SVM score in cell PPD. The M1 analog was created by replacing alanine with leucine on the 15th. The M2 analog was designed by substituting alanine with leucine and isoleucine with arginine at the 15th and 17th positions. According to the Gromacs results, the M2 peptide indicated more stability. RMSD and RMSF results showed no undesirable fluctuations during the 200 ns MD simulation. The MIC and MBC values for the M1 peptide were calculated in a range of 8–128 μg/ml, while the M2 peptide limited the bacterial growth to 32–128 μg/mL. Both peptides indicated less toxicity than natural MLT, based on MTT assay results. The hemolytic activity of the M1 analog was more than M2 at 16 μg/mL concentration. M1 peptide displayed the highest selectivity index against S. aureus and A. baumannii, which were approximately 5.27-fold improvements compared to MLT. In conclusion, we introduced two analogs of MLT with low toxicity, low hemolytic activity, and higher stability, along with retaining antimicrobial properties against gram-negative and positive bacteria.






Similar content being viewed by others
Abbreviations
- AMPs:
-
Antimicrobial peptides
- M1:
-
GLPLLISWIKRKRQQ
- M2:
-
GLPLLRSWIKRKRQQ
- GRAVY index:
-
The sum of the hydropathy values
- D:
-
Discrimination factor
- H:
-
Mean hydrophobicity
- μH:
-
The hydrophobic moment
- z:
-
Net charge
- NPT:
-
Constant pressure
- NVT:
-
Constant volume
- RMSF:
-
Root-mean-square fluctuations
- RMSD:
-
Root-mean-square deviation
- DPPC:
-
Dipalmitoyl phosphatidylcholine membrane
References
Karyne R, Lechuga GC, Souza ALA, da Silva Carvalho JPR, Bôas MHSV, De Simone SG (2020) Pan-drug resistant acinetobacter baumannii, but not other strains, are resistant to the bee venom peptide mellitin. Antibiotics 9(4):178
Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS et al (2019) Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 19(1):56–66
Koehbach J, Craik DJ (2019) The vast structural diversity of antimicrobial peptides. Trends Pharmacol Sci 40(7):517–528
Nuti R, Goud NS, Saraswati AP, Alvala R, Alvala M (2017) Antimicrobial peptides: a promising therapeutic strategy in tackling antimicrobial resistance. Curr Med Chem 24(38):4303–4314
Dosler S, Karaaslan E (2014) Inhibition and destruction of Pseudomonas aeruginosa biofilms by antibiotics and antimicrobial peptides. Peptides 62:32–37
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415(6870):389–395
Memariani H, Memariani M, Moravvej H, Shahidi-Dadras M (2020) Melittin: a venom-derived peptide with promising anti-viral properties. Eur J Clin Microbiol Infect Dis 39(1):5–17
Wimley WC (2010) Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol 5(10):905–917
Rady I, Siddiqui IA, Rady M, Mukhtar H (2017) Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett 402:16–31
Terwilliger TC, Eisenberg D (1982) The structure of melittin. II. Interpretation of the structure. J Biol Chem 257(11):6016–6022
Tacón A (2016) Melittin and cancer. Cancer Cells 8:10–12
Akbari R, Hakemi-Vala M, Pashaie F, Bevalian P, Hashemi A, Pooshang Bagheri K (2019) Highly synergistic effects of melittin with conventional antibiotics against multidrug-resistant isolates of acinetobacter baumannii and pseudomonas aeruginosa. Microb Drug Resist 25(2):193–202
Pashaei F, Bevalian P, Akbari R, Bagheri KP (2019) Single dose eradication of extensively drug resistant Acinetobacter spp. in a mouse model of burn infection by melittin antimicrobial peptide. Microb Pathog 127:60–69
Farajnia S, Rahbarnia L, Khajehnasiri N, Zarredar H (2022) Design of a hybrid peptide derived from Melittin and CXCL14–C17: a molecular dynamics simulation study. Biologia 1–12
Akbarzadeh-Khiavi M, Torabi M, Olfati A-H, Rahbarnia L, Safary A (2022) Bio-nano scale modifications of melittin for improving therapeutic efficacy. Expert Opin Biol Ther (just-accepted)
Juvvadi P, Vunnam S, Merrifield R (1996) Synthetic melittin, its enantio, retro, and retroenantio isomers, and selected chimeric analogs: their antibacterial, hemolytic, and lipid bilayer action. J Am Chem Soc 118(38):8989–8997
Oren Z, Shai Y (1997) Selective lysis of bacteria but not mammalian cells by diastereomers of melittin: structure− function study. Biochemistry 36(7):1826–1835
Subbalakshmi C, Nagaraj R, Sitaram N (1999) Biological activities of C-terminal 15-residue synthetic fragment of melittin: design of an analog with improved antibacterial activity. FEBS Lett 448(1):62–66
Gautam A, Chaudhary K, Kumar R, Raghava GP (2015) Computer-aided virtual screening and designing of cell-penetrating peptides. Methods Mol Biol (Clifton, NJ) 1324:59–69
Gautam A, Chaudhary K, Kumar R, Sharma A, Kapoor P, Tyagi A et al (2013) In silico approaches for designing highly effective cell penetrating peptides. J Transl Med 11:1–12
Gautier R, Douguet D, Antonny B, Drin G (2008) HELIQUEST: a web server to screen sequences with specific α-helical properties. Bioinformatics 24(18):2101–2102
Doytchinova IA, Flower DR (2007) VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics 8(1):4
Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27(3):343–350
Rasafar N, Barzegar A, Aghdam EM (2020) Design and development of high affinity dual anticancer peptide-inhibitors against p53-MDM2/X interaction. Life Sci 245:117358
Zarrinnahad H, Mahmoodzadeh A, Hamidi MP, Mahdavi M, Moradi A, Bagheri KP et al (2018) Apoptotic effect of melittin purified from Iranian honey bee venom on human cervical cancer HeLa Cell Line. Int J Pept Res Ther 24(4):563–570
Lotfi F, Shojaie M, Rahbarnia L, Dehnad A, Naghili B, Lotfi H (2022) Molecular characterization and genetic diversity of multidrug-and extensively drug-resistant A. baumannii clinical isolates. Gene Rep 26:101455
Ghavghani FR, Rahbarnia L, Naghili B, Dehnad A, Bazmani A, Varshochi M et al (2019) Nasal and extra nasal MRSA colonization in hemodialysis patients of north-west of Iran. BMC Res Notes 12(1):1–5
Weinstein MP, Lewis JS (2020) The clinical and laboratory standards institute subcommittee on antimicrobial susceptibility testing: background, organization, functions, and processes. J Clin Microbiol 58(3):e01864-e1919
Silva S, Alves N, Silva P, Vieira T, Maciel P, Castellano LR et al (2019) Antibacterial Activity of Rosmarinus officinalis, Zingiber officinale, Citrus aurantium bergamia, and Copaifera officinalis alone and in combination with calcium hydroxide against Enterococcus faecalis. Biomed Res Int 2019
Eatemadi A, Al Risi E, Kasliwal A, Al Zaˊabi A, Moradzadegan H, Aslani Z (2021) A proposed evidence-based local guideline for definition of multidrug-resistant (MDR), extensively drug-resistant (XDR) and pan drug-resistant (PDR) bacteria by the microbiology laboratory. Int J Curr Sci Res Rev 4
Al-Badri ZM, Som A, Lyon S, Nelson CF, Nüsslein K, Tew GN (2008) Investigating the effect of increasing charge density on the hemolytic activity of synthetic antimicrobial polymers. Biomacromol 9(10):2805–2810
Petersson EJ (2021) Synthetic and enzymatic modifications of the peptide backbone: Academic Press
Leandro LF, Mendes CA, Casemiro LA, Vinholis AH, Cunha WR, Almeida Rd et al (2015) Antimicrobial activity of apitoxin, melittin and phospholipase A 2 of honey bee (Apis mellifera) venom against oral pathogens. An Acad Bras Cienc 87:147–155
Parvekar P, Palaskar J, Metgud S, Maria R, Dutta S (2020) The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater Investig Dent 7(1):105–109
Askari P, Namaei MH, Ghazvini K, Hosseini M (2021) In vitro and in vivo toxicity and antibacterial efficacy of melittin against clinical extensively drug-resistant bacteria. BMC Pharmacol Toxicol 22(1):1–12
Giacometti A, Cirioni O, Kamysz W, D’Amato G, Silvestri C, Del Prete MS et al (2003) Comparative activities of cecropin A, melittin, and cecropin A-melittin peptide CA(1–7)M(2–9)NH2 against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. Peptides 24(9):1315–1318
Lázár V, Martins A, Spohn R, Daruka L, Grézal G, Fekete G et al (2018) Antibiotic-resistant bacteria show widespread collateral sensitivity to antimicrobial peptides. Nat Microbiol 3(6):718–731
Acknowledgements
This study was supported by the Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Author information
Authors and Affiliations
Contributions
L.R. designed the study and revised the manuscript; A.S. performed the data analysis and interpretation. P.M. and A.Sh wrote the first draft of the manuscript. Z.M. discussed the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study was conducted after ethical approval of the ethics committee of Tabriz University of medical science, Tabriz Iran (reference number: IR.TBZMED.REC.1401.121). Data and clinical samples were collected after written consent with a brief description about the importance of the study to the participants
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rad, P.M., Rahbarnia, L., Safary, A. et al. The Synthetic Antimicrobial Peptide Derived From Melittin Displays Low Toxicity and Anti-infectious Properties. Probiotics & Antimicro. Prot. 16, 490–500 (2024). https://doi.org/10.1007/s12602-023-10066-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-023-10066-6