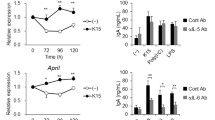
Abstract
Streptococcus salivarius BLIS K12 is a probiotic strain developed for application to the oral cavity. The strain was originally characterised for its in vitro antibacterial activity against the prominent oral pathogen Streptococcus pyogenes. More recent research has expanded its applications to include reducing halitosis, preventing otitis media and protecting against virus infections of the respiratory tract. A potential mechanism for this anti-viral activity could be the stimulation of salivary interferon gamma (IFN-γ) production in the oral cavity. The aim of this study was to investigate whether the ingestion of and oral cavity colonisation by S. salivarius BLIS K12 is associated with enhancement of IFN-γ levels in saliva. Application of ELISA demonstrated that consumption of S. salivarius BLIS K12 effected an increase in salivary IFN-γ, and this response was more consistent with use of viable cells than following ingestion of heat-killed S. salivarius BLIS K12. Interestingly, those subjects who more successfully colonised with S. salivarius BLIS K12 did not experience a relatively larger increase in their IFN-γ levels, indicating that the observed IFN-γ response occurs independently of colonisation efficacy. In summary, the consumption of S. salivarius BLIS K12 increases salivary levels of IFN-γ, an effect that may contribute to protection of the host against certain virus infections.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Hegarty JW, Guinane CM, Ross RP et al (2016) Bacteriocin production: a relatively unharnessed probiotic trait? https://doi.org/10.12688/f1000research.9615.1
Anderson RC, Cookson AL, McNabb WC et al (2010) Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol 10:1–11. https://doi.org/10.1186/1471-2180-10-316/FIGURES/4
Kechagia M, Basoulis D, Konstantopoulou S et al (2013) Health benefits of probiotics: a review. ISRN Nutr 2013:1–7. https://doi.org/10.5402/2013/481651
Ljungh Å, Wadström T (2006) Lactic acid bacteria as probiotics. Curr Issues Intestinal Microbiol 7:73–90
Tagg JR, Dierksen KP (2003) Bacterial replacement therapy: adapting ‘germ warfare’ to infection prevention. Trends Biotechnol 21(5):217–223. https://doi.org/10.1016/S0167-7799(03)00085-4
Tagg JR (2004) Prevention of streptococcal pharyngitis by anti-Streptococcus pyogenes bacteriocin-like inhibitory substances (BLIS) produced by Streptococcus salivarius. Indian J Microbiol 119:13
Burton JP, Chilcott CN, Wescombe PA, Tagg JR (2010) Extended safety data for the oral cavity probiotic Streptococcus salivarius K12. Probiotics Antimicrob Proteins 2:135–144. https://doi.org/10.1007/s12602-010-9045-4
Ishijima SA, Hayama K, Burton JP et al (2012) Effect of Streptococcus salivarius K12 on the in vitro growth of Candida albicans and its protective effect in an oral candidiasis model. Appl Environ Microbiol 78:2190–2199. https://doi.org/10.1128/AEM.07055-11
Laws GL, Hale JDF, Kemp RA (2021) Human systemic immune response to ingestion of the oral probiotic Streptococcus salivarius BLIS K12. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-021-09822-3
Cosseau C, Devine DA, Dullaghan E et al (2008) The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun 76:4163–4175
MacDonald KW, Chanyi RM, Macklaim JM et al (2021) Streptococcus salivarius inhibits immune activation by periodontal disease pathogens. BMC Oral Health 21:245. https://doi.org/10.1186/s12903-021-01606-z
Hiscott J, Kwon H, Génin P (2001) Hostile takeovers: viral appropriation of the NF-kB pathway. J Clin Investig 107:143. https://doi.org/10.1172/JCI11918
Wang Q, Lin X, Xiang X et al (2021) Oropharyngeal probiotic ENT-K12 prevents respiratory tract infections among frontline medical staff fighting against COVID-19: a pilot study. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2021.646184
di Pierro F, Colombo M (2021) The administration of S. salivarius K12 to children may reduce the rate of SARS-CoV-2 infection. Minerva Med 112. https://doi.org/10.23736/S0026-4806.21.07487-5
di Pierro F, Colombo M, Zanvit A, Rottoli AS (2016) Positive clinical outcomes derived from using Streptococcus salivarius K12 to prevent streptococcal pharyngotonsillitis in children: a pilot investigation. Drug Healthc Patient Saf 8:77–81. https://doi.org/10.2147/DHPS.S117214
Guo H, Xiang X, Lin X et al (2022) Oropharyngeal probiotic ENT-K12 as an effective dietary intervention for children with recurrent respiratory tract infections during cold season. Front Nutr. https://doi.org/10.3389/fnut.2022.900448
Chilcott C, Crowley L, Kulkarni V et al (2005) Elevated levels of interferon gamma in human saliva following ingestion of Streptococcus salivarius K12. NZMS, Dunedin, New Zealand 22-25 November 2005
Schroder K, Hertzog PJ, Ravasi T, Hume DA (2004) Interferon-y: an overview of signals, mechanisms and functions. J Leukoc Biol 75:163–189. https://doi.org/10.1189/jlb.0603252.Journal
Hu X, Herrero C, Li W-P et al (2002) Sensitization of IFN-gamma Jak-STAT signaling during macrophage activation. Nat Immunol 3:859–866. https://doi.org/10.1038/ni828
Lowenstein CJ, Alley EW, Raval P et al (1993) Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon y and lipopolysaccharide. Biochemistry 90:9730–9734. https://doi.org/10.1073/pnas.90.20.9730
Xie QW, Cho HJ, Calaycay J et al (1979) (1992) Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256:225–228. https://doi.org/10.1126/SCIENCE.1373522
Tagg JR, Read RS, McGiven AR (1973) Bacteriocin of a group A streptococcus: partial purification and properties. Antimicrob Agents Chemother 4:214–221. https://doi.org/10.1128/aac.4.3.214
Morse SA (1996) Medical Microbiology 4th edition 12
Harris DP, Haynes L, Sayles PC et al (2000) Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 1:475–482
Bouwer AL, Saunderson SC, Dunn AC et al (2013) Rapid interferon-gamma release from natural killer cells induced by a streptococcal commensal. 33:459–466. https://doi.org/10.1089/JIR.2012.0116
MacDonald K, Chanyi R, Macklaim J et al (2021) Streptococcus salivarius inhibits immune activation by periodontal disease pathogens. BMC Oral Health. https://doi.org/10.1186/S12903-021-01606-Z
Burton J, Chilcott C, Moore C et al (2006) A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. J Appl Microbiol 100:754–764. https://doi.org/10.1111/J.1365-2672.2006.02837.X
Wescombe PA, Heng NCK, Burton JP et al (2009) Streptococcal bacteriocins and the case for Streptococcus salivarius as model oral probiotics. Future Microbiol 4:819–835
Hyink O, Wescombe PA, Upton M et al (2007) Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl Environ Microbiol. https://doi.org/10.1128/AEM.02265-06
Burton JP, Cowley S, Simon RR et al (2011) Evaluation of safety and human tolerance of the oral probiotic Streptococcus salivarius K12: a randomized, placebo-controlled, double-blind study. Food Chem Toxicol 49:2356–2364. https://doi.org/10.1016/j.fct.2011.06.038
Author information
Authors and Affiliations
Contributions
G.A.L. helped design the study, and ran the clinical trial and laboratory analysis. L.K.H. wrote the main manuscript text. J.R.T. contributed to the planning and assessment of results. J.D.F.H. designed the project and reviewed the results. All authors reviewed and contributed to writing.
Corresponding author
Ethics declarations
Ethics
Health and Disability Ethics Committee LRS/09/02/002/AM03.
Conflict of Interest
G.A.L., L.K.H., J.R.T. and J.D.F.H are/or were employees of Blis Technologies at the time of this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Laws, G.A., Harold, L.K., Tagg, J.R. et al. Interferon Gamma Response in Human Saliva Following Exposure to the Oral Probiotic Streptococcus salivarius BLIS K12. Probiotics & Antimicro. Prot. 16, 93–98 (2024). https://doi.org/10.1007/s12602-022-10010-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-10010-0