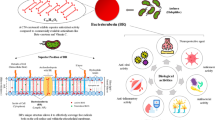
Abstract
The emergence of antibiotic resistance poses a serious and challenging threat to healthcare systems, making it imperative to discover novel therapeutic options. This work reports the isolation and characterization of a thermostable trypsin inhibitor from chia (Salvia hispanica L.) seeds, with antibacterial activity against Staphylococcus aureus sensitive and resistant to methicillin. The trypsin inhibitor ShTI was purified from chia seeds through crude extract heat treatment, followed by affinity and reversed-phase chromatography. Tricine-SDS–PAGE revealed a single glycoprotein band of ~ 11 kDa under nonreducing conditions, confirmed by mass spectrometry analysis (11.558 kDa). ShTI was remarkably stable under high temperatures (100 °C; 120 min) and a broad pH range (2–10; 30 min). Upon exposure to DTT (0.1 M; 120 min), ShTI antitrypsin activity was partially lost (~ 38%), indicating the participation of disulfide bridges in its structure. ShTI is a competitive inhibitor (Ki = 1.79 × 10–8 M; IC50 = 1.74 × 10–8 M) that forms a 1:1 stoichiometry ratio for the ShTI:trypsin complex. ShTI displayed antibacterial activity alone (MICs range from 15.83 to 19.03 µM) and in combination with oxacillin (FICI range from 0.20 to 0.33) against strains of S. aureus, including methicillin-resistant strains. Overproduction of reactive oxygen species and plasma membrane pore formation are involved in the antibacterial action mode of ShTI. Overall, ShTI represents a novel candidate for use as a therapeutic agent for the bacterial management of S. aureus infections.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are available from the corresponding author upon reasonable request.
References
Church NA, McKillip JL (2021) Antibiotic resistance crisis: challenges and imperatives. Biologia 76(5):1535–1550. https://doi.org/10.1007/s11756-021-00697-x
Ventola CL (2015) The antibiotic resistance crisis: part 1: causes and threats. Pharm Ther 40(4):277–283
Abushaheen MA, Muzaheed FAJ, Alosaimi M, Mansy W, George M, Acharya S, Rathod S, Divakar DD, Jhugroo C, Vellappally S, Khan AA, Shaik J, Jhugroo P (2020) Antimicrobial resistance, mechanisms and its clinical significance. Dis Mon 66(6):100971. https://doi.org/10.1016/j.disamonth.2020.100971
Pulingam T, Parumasivam T, Gazzali AM, Sulaimana AM, Chee JY, Lakshmanan M, Chin CF, Sudesh K (2021) Antimicrobial resistance: prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur J Pharm Sci 170:106103. https://doi.org/10.1016/j.ejps.2021.106103
Cunha BR, Fonseca LP, Calado CRC (2019) Antibiotic discovery: where have we come from, where do we go? Antibiotics (Basel) 8(2):45. https://doi.org/10.3390/antibiotics8020045
Butler MS, Gigante V, Sati H, Paulin S, Al-Sulaiman L, Rex JH, Fernandes P, Arias CA, Paul M, Thwaites GE, Czaplewski L, Alm RA, Lienhardt C, Spigelman M, Silver LL, Ohmagari N, Kozlov R, Harbarth S, Beyer P (2022) Analysis of the clinical pipeline of treatments for drug resistant bacterial infections: despite progress, more action is needed. Antimicrob Agents Chemother 66(3):e01991-e2021. https://doi.org/10.1128/aac.01991-21
Nascimento AMS, Oliveira Segundo VH, Aguiar AJFC, Piuvezam G, Passos TS, Damasceno KSFSC, Morais AHA (2022) Antibacterial action mechanisms and mode of trypsin inhibitors: a systematic review. J Enzyme Inhib Med Chem 37(1):749–759. https://doi.org/10.1080/14756366.2022.2039918
Oliveira CFR, Oliveira CT, Taveira GB, Mello EO, Gomes VM, Macedo MLR (2018) Characterization of a Kunitz trypsin inhibitor from Enterolobium timbouva with activity against Candida species. Int J Biol Macromol 119:645–653. https://doi.org/10.1016/j.ijbiomac.2018.07.034
Clemente M, Corigliano MG, Pariani SA, Sánchez-López EF, Sander VA, Ramos-Duarte VA (2019) Plant serine protease inhibitors: biotechnology application in agriculture and molecular farming. Int J Mol Sci 20(6):1345. https://doi.org/10.3390/ijms20061345
Hellinger R, Gruber CW (2019) Peptide-based protease inhibitors from plants. Drug Discov Today 24(9):1877–1889. https://doi.org/10.1016/j.drudis.2019.05.026
Cotabarren J, Lufrano D, Parisi MG, Obregón WD (2020) Biotechnological, biomedical, and agronomical applications of plant protease inhibitors with high stability: A systematic review. Plant Sci 292:110398. https://doi.org/10.1016/j.plantsci.2019.110398
Grancieri M, Martino HSD, Gonzalez de Mejia E (2019) Chia seed (Salvia hispanica L.) as a source of proteins and bioactive peptides with health benefits: a review. Compr Rev Food Sci Food Saf 18(2):480–499. https://doi.org/10.1111/1541-4337.12423
Motyka S, Koc K, Ekiert H, Blicharska E, Czarnek K, Szopa A (2022) The current state of knowledge on Salvia hispanica and Salviae hispanicae semen (Chia Seeds). Molecules 27(4):1207. https://doi.org/10.3390/molecules27041207
Timilsena YP, Adhikari R, Barrow CJ, Adhikari B (2016) Physicochemical and functional properties of protein isolate produced from Australian chia seeds. Food Chem 212:648–656. https://doi.org/10.1016/j.foodchem.2016.06.017
Aguilar-Toalá JE, Deering AJ, Liceaga AM (2020) New insights into the antimicrobial properties of hydrolysates and peptide fractions derived from chia seed (Salvia hispanica L.). Probiotics Antimicrob Proteins 12(4):1571–1581. https://doi.org/10.1007/s12602-020-09653-8
Grancieri M, Martino HSD, de Mejia EG (2019) Digested total protein and protein fractions from chia seed (Salvia hispanica L.) had high scavenging capacity and inhibited 5-LOX, COX-1-2, and iNOS enzymes. Food Chem 289:204–214. https://doi.org/10.1016/j.foodchem.2019.03.036
Cotabarren J, Rosso AM, Tellechea M, García-Pardo J, Rivera JL, Obregón WD, Parisi MG (2019) Adding value to the chia (Salvia hispanica L.) expeller: Production of bioactive peptides with antioxidant properties by enzymatic hydrolysis with Papain. Food Chem 274:848–856. https://doi.org/10.1016/j.foodchem.2018.09.061
Ozón B, Cotabarren J, Valicenti T, Parisi MG, Obregón WD (2022) Chia expeller: a promising source of antioxidant, antihypertensive and antithrombotic peptides produced by enzymatic hydrolysis with Alcalase and Flavourzyme. Food Chem 380:132185. https://doi.org/10.1016/j.foodchem.2022.132185
Souza APA, Nascimento LMAM, Lima VCO, Carvalho FMC, Santos EA, Morais AHA (2017) Atividade antitríptica em semente e produto alimentício de chia (Salvia hispanica L.). DEMETRA Aliment Nutr Saúde 12(1):319–331. https://doi.org/10.12957/demetra.2017.25636
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Erlanger BF, Kokowsky N, Cohen W (1961) The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95:271–278. https://doi.org/10.1016/0003-9861(61)90145-X
Schägger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166(2):368–379. https://doi.org/10.1016/0003-2697(87)90587-2
Zacharius RM, Zell TE, Morrison JH, Woodlock JJ (1969) Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem 30(1):148–152. https://doi.org/10.1016/0003-2697(69)90383-2
Costa HPS, Oliveira JTA, Sousa DOB, Morais JKS, Moreno FB, Monteiro-Moreira ACO, Viegas RA, Vasconcelos IM (2014) JcTI-I: a novel trypsin inhibitor from Jatropha curcas seed cake with potential for bacterial infection treatment. Front Microbiol 5:5. https://doi.org/10.3389/fmicb.2014.00005
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56(3):658–666. https://doi.org/10.1021/ja01318a036
Dixon M (1953) The determination of enzyme inhibitor constants. Biochem J 55(1):170–171. https://doi.org/10.1042/bj0550170
Clinical and Laboratory Standards Institute (2015) Reference Method for Diluition Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard M07-A10, 10th ed., Wayne, PA. https://clsi.org/media/1632/m07a10_sample.pdf
Liu B, Huang H, Yang Z, Liu B, Gou S, Zhong C, Han X, Zhang Y, Ni J, Wang R (2017) Design of novel antimicrobial peptide dimer analogues with enhanced antimicrobial activity in vitro and in vivo by intermolecular triazole bridge strategy. Peptides 88:115–125. https://doi.org/10.1016/j.peptides.2016.12.016
Odds FC (2003) Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52(1):1. https://doi.org/10.1093/jac/dkg301
Martins TF, Vasconcelos IM, Silva RGG, Silva FDA, Souza PFN, Varela ALN, Albuquerque LM, Oliveira JTA (2018) A Bowman-Birk inhibitor from the seeds of Luetzelburgia auriculata inhibits Staphylococcus aureus growth by promoting severe cell membrane damage. J Nat Prod 81(7):1497–1507. https://doi.org/10.1021/acs.jnatprod.7b00545
Din Z, Alam M, Ullah H, Shi D, Xu B, Li H, Xiao C (2021) Nutritional, phytochemical and therapeutic potential of chia seed (Salvia hispanica L.). A mini-review. Food Hydrocoll 1:100010. https://doi.org/10.1016/j.fhfh.2021.100010
Alassa V, Ferreira MDR, Villafañe N, D’Alessandro ME (2022) α-Linolenic acid rich-chia seed modulates visceral adipose tissue collagen deposition, lipolytic enzymes expression, insulin signaling and GLUT-4 levels in a diet-induced adiposity rodent model. Food Res Int 156:111164. https://doi.org/10.1016/j.foodres.2022.111164
Grancieri M, Verediano TA, Sant’Ana CT, Assis A, Toledo RL, Mejia EG, Martino HSD (2022) Digested protein from chia seed (Salvia hispanica L) prevents obesity and associated inflammation of adipose tissue in mice fed a high-fat diet. PharmaNutrition 21:100298. https://doi.org/10.1016/j.phanu.2022.100298
Fadwa EQ, Amssayef A, Eddouks M (2022) Antihyperglycemic and antidyslipidemic activities of the aqueous Salvia hispanica extract in diabetic rat. Cardiovasc Hematol Agents Med Chem 20(1):60–66. https://doi.org/10.2174/1871525719666210112154340
Madrazo AL, Ortíz ABF, Mendoza LFM, Campos MRS (2022) Antibacterial peptide fractions from chia seeds (Salvia hispanica L.) and their stability to food processing conditions. J Food Sci Technol 1–9. https://doi.org/10.1007/s13197-022-05506-0
Ahmed AZ, Mumbrekar KD, Satyam SM, Shetty P, D’Souza MR, Singh VK (2021) Chia seed oil ameliorates doxorubicin-induced cardiotoxicity in female wistar rats: an electrocardiographic, biochemical and histopathological approach. Cardiovasc Toxicol 21(7):533–542. https://doi.org/10.1007/s12012-021-09644-3
Villanueva-Lazo A, De la Paz SM, Grao-Cruces E, Pedroche J, Toscano R, Milian F, Milian-Linares MC (2022) Antioxidant and immunomodulatory properties of chia protein hydrolysates in primary human monocyte–macrophage plasticity. Foods 11(5):623. https://doi.org/10.3390/foods11050623
Lovato AC, Corgozinho MLMV, Alves LV, Martins SR, Duarte RCF, Cardoso CN, Silvino JPP, Ferreira CN, Silva IFO, Mota APL (2022) Effect of the use of Chia (Salvia hispanica L.) seeds on antioxidant status and anthropometric parameters in obese, type 2 diabetics and/or hypertensive patients. Res Soc Dev 11(4):e46511427432. https://doi.org/10.33448/rsd-v11i4.27432
Villanueva-Lazo A, De la Paz SM, Rodriguez-Martin NM, Millan F, Carrera C, Pedroche JJ, Millan-Linares MC (2021) Antihypertensive and antioxidant activity of chia protein techno-functional extensive hydrolysates. Foods 10(10):2297. https://doi.org/10.3390/foods10102297
Moreira LPD, Enes BN, de São José VPB, Toledo RCL, Ladeira LCM, Cardoso RR, Duarte VS, Hermsdorff HHM, De Barros FAR, Martino HSD (2022) Chia (Salvia hispanica L.) Flour and oil ameliorate metabolic disorders in the liver of rats fed a high-fat and high fructose diet. Foods 11(3):285 https://doi.org/10.3390/foods11030285
Geetha KM, Shankar J, Wilson B (2022) Neuroprotective effect of chia seed oil nanoemulsion against rotenone induced motor impairment and oxidative stress in mice model of Parkinson’s disease. Adv Trad Med 1–18. https://doi.org/10.1007/s13596-022-00648-0
Srikanth S, Chen Z (2016) Plant protease inhibitors in therapeutics-focus on cancer therapy. Front Pharmacol 7:470. https://doi.org/10.3389/fphar.2016.00470
Silva RGG, Vasconcelos IM, Filho AJUB, Carvalho AFU, Souza TM, Gondim DMF, Varela ALN, Oliveira JTA (2015) Castor bean cake contains a trypsin inhibitor that displays antifungal activity against Colletotrichum gloeosporioides and inhibits the midgut proteases of the dengue mosquito larvae. Ind Crops Prod 70:48–55. https://doi.org/10.1016/j.indcrop.2015.02.058
Cotabarren J, Broitman DJ, Quiroga E, Obregón WD (2020) GdTI, the first thermostable trypsin inhibitor from Geoffroea decorticans seeds. A novel natural drug with potential application in biomedicine. Int J Biol Macromol 148:869–879. https://doi.org/10.1016/j.ijbiomac.2020.01.214
Ee KY, Zhao J, Rehman A, Agboola S (2011) Glycosylation, amino acid analysis and kinetic properties of a major Kunitz-type trypsin inhibitor from Acacia victoriae Bentham seeds. Food Chem 129(3):1224–1227. https://doi.org/10.1016/j.foodchem.2011.05.026
Dias LP, Oliveira JTA, Rocha-Bezerra LCB, Sousa DOB, Costa HPS, Araujo NMS, Carvalho AFU, Tabosa PMS, Monteiro-Moreira ACO, Lobo MDP, Moreno FBMB, Rocha BAM, Lopes JLS, Beltramini LM, Vasconcelos IM (2017) A trypsin inhibitor purified from Cassia leiandra seeds has insecticidal activity against Aedes aegypti. Process Biochem 57:228–238. https://doi.org/10.1016/j.procbio.2017.03.015
Joshi RS, Mishra M, Suresh CG, Gupta VS, Giri AP (2013) Complementation of intramolecular interactions for structural-functional stability of plant serine proteinase inhibitors. Biochim Biophys Acta 11:5087–5094. https://doi.org/10.1016/j.bbagen.2013.07.019
Silva Bezerra C, Oliveira CFR, Machado OLT, Mello GSV, Rocha Pitta MG, Melo Rêgo MJB, Napoleão TH, Paiva PMG, Ribeiro SFF, Gomes VM, Silva ON, Maria-Neto S, Franco OL, Macedo MLR (2016) Exploiting the biological roles of the trypsin inhibitor from Inga vera seeds: a multifunctional Kunitz inhibitor. Process Biochem 51(6):792–803. https://doi.org/10.1016/j.procbio.2016.03.008
Barros KMA, Sardi JCO, Maria-Neto S, Macedo AJ, Ramalho SR, Oliveira DGL, Pontes GS, Weber SS, Oliveira CFR, Macedo MLR (2021) A new Kunitz trypsin inhibitor from Erythrina poeppigiana exhibits antimicrobial and antibiofilm properties against bacteria. Biomed Pharmacother 144:112198. https://doi.org/10.1016/j.biopha.2021.112198
Bacha AB, Jemel I, Moubayed NMS, Abdelmalek IB (2017) Purification and characterization of a newly serine protease inhibitor from Rhamnus frangula with potential for use as therapeutic drug. 3 Biotech 7(2):148. https://doi.org/10.1007/s13205-017-0764-z
Miller BW, Torres JP, Tun JO, Flores MS, Forteza I, Rosenberg G, Haygood MG, Schmidt EW, Concepcion GP (2020) Synergistic anti-methicillin-resistant Staphylococcus aureus (MRSA) activity and absolute stereochemistry of 7, 8-dideoxygriseorhodin C. J Antibiot 73(5):290–298. https://doi.org/10.1038/s41429-019-0275-8
World Health Organization (2017) Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. WHO Press, Geneva, pp 1–7
Funding
The authors thank the Brazilian institutions CNPq (National Council for Scientific and Technological Development – Award number: 431827/2016–8), CAPES (Coordination of Improvement of Higher Education, and Drug Research and Development Center—Federal University of Ceará (NPDM-UFC) for physical installation and financial support of this research. We also gratefully acknowledge the Center of Advanced Microscopy and Microanalysis (Central Analytical Facility) at the Federal University of Ceará, Brazil, for the scanning electron microscopy (SEM) analysis.
Author information
Authors and Affiliations
Contributions
Adson Ávila de Souza: conceptualization, methodology design, validation, investigation, data curation, formal analysis, writing—original draft and writing—review and editing. Adrianne Maia Lima: investigation. Daniele de Oliveira Bezerra de Sousa: funding acquisition, writing—initial draft and writing—review and editing. Francisca Cristiane Nogueira: investigation. José Carlos do Sacramento Neto: investigation. Lucas Pinheiro Dias: research and formal analysis. Nadine Monteiro Salgueiro Araújo: investigation and formal analysis. Celso Shiniti Nagano: investigation, formal analysis, funding acquisition, writing—original draft and writing—review and editing. Hélio Vitoriano Nobre Júnior: funding acquisition, writing—initial draft and writing—review and editing. Cecília Rocha da Silva: funding acquisition, writing—original draft and writing—review and editing. Lívia Gurgel do Amaral Valente Sá: investigation and formal analysis. João Batista de Andrade Neto: writing—initial draft and writing—review and editing. Fátima Daiana Dias Barroso: investigation and formal analysis. Maria Elisabete Amaral de Moraes: funding acquisition, writing—original draft and writing—review and editing. Hermógenes David de Oliveira: conceptualization, formal analysis, data curation, supervision, funding acquisition, writing—initial draft and writing—review and editing.
Corresponding author
Ethics declarations
Ethics Approval
No approval from research ethics committees was required to achieve the goals of this study because experimental work was conducted with bacterial strains.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Souza, A.Á., Lima, A.M., dede Oliveira BezerraSousa, D. et al. Chia (Salvia hispanica L.) Seeds Contain a Highly Stable Trypsin Inhibitor with Potential for Bacterial Management Alone or in Drug Combination Therapy with Oxacillin. Probiotics & Antimicro. Prot. 15, 1221–1233 (2023). https://doi.org/10.1007/s12602-022-09979-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-09979-5