Abstract
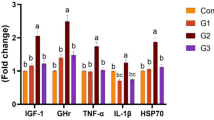
The present study investigated the effects of combined and singular oral administration of Bio-Aqua® with different dosages of sodium diformate (NaDF) on biochemical indices, innate immune responses, antioxidant effects, and expressions of immunological related genes of Caspian brown trout (Salmo trutta caspius). Fingerlings Salmo trutta caspius (n = 1800; initial weight 15 ± 3 g) were randomly allocated into five groups (120 fish group-1 in triplicates). Control diet: without any addition, G1, G2, G3, and G4 received diets containing 0.2 g kg−1 commercial probiotic Bio-Aqua® combined with 0, 0.5, 1.0, and 1.5% NaDF to the basal diet for 60 days according to recommended dosages reported in previous studies. Results indicated that serum bactericidal activity (G3 on day 60 and G1 on day 30) and classic complement in all groups (on day 60) (G1 and G2 on day 30) were significantly elevated (P < 0.05). The serum lysozyme, glucose, globulin, and albumin levels showed no significant differences between all groups compared to the control group (P > 0.05). On days 30 and 60 of the sampling, no significant difference was observed in the amount of superoxide disotase (SOD) and catalase (CAT) between the treatments (P > 0.05) but activity of malondialdehyde (MDA) was lower in G1 than the control (P < 0.05). The expression of the immune-regulating genes IL-10, IL-1β, GTP, FATP, and IGF was significantly improved in all probiotic + acidifier–treated groups (P < 0.05). The current findings showed that mixture of Bio-Aqua® and NaDF (1.5% + pro) is beneficial, as it effectively improves some immune parameters and expression of immunological and growth-related genes in Caspian brown trout.




Similar content being viewed by others
Data Availability
The data supporting the study’s conclusions are accessible upon request from the corresponding author. Due to privacy and ethical concerns, the data is not publicly accessible.
References
Tacon AG, Metian M (2013) Fish matters: importance of aquatic foods in human nutrition and global food supply. Rev Fish Sci Aquac 21:22–38. https://doi.org/10.1080/10641262.2012.753405
Mohammadian T, Momeni H, Mesbah M, Tabandeh MR, Khosravi M (2020) Effect of different levels of dietary acidifier “sodium diformate” on the innate immune system and expression of growth and immunological related genes in Salmo trutta caspius. Aquac Nutr 26:2074–2085. https://doi.org/10.1111/anu.13148
Adel M, Safari R, Pourgholam R, Zorriehzahra J, Esteban MÁ (2015) Dietary peppermint (Mentha piperita) extracts promote growth performance and increase the main humoral immune parameters (both at mucosal and systemic level) of Caspian brown trout (Salmo trutta caspius Kessler, 1877). Fish Shellfish Immunol 47:623–629. https://doi.org/10.1016/j.fsi.2015.10.005
Jobling M (2012) National Research Council (NRC): nutrient requirements of fish and shrimp. Springer. https://doi.org/10.1007/s10499-011-9480-6
Zhou Q, Li K, Jun X, Bo L (2009) Role and functions of beneficial microorganisms in sustainable aquaculture. Bioresour Technol 100:3780–3786. https://doi.org/10.1016/j.biortech.2008.12.037
Dawood MA, Koshio S, Esteban MÁ (2018) Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquac 10:950–974. https://doi.org/10.1111/raq.12209
FAO/WHO (2002) Guidelines for the evaluation of probiotics in food. Food and Agriculture Organization of the United Nations and World Health Organization Working Group Report. Rome: Food and Agriculture Organization
Mamun M, Nasren S, Rathore S, Sidiq M, Dharmakar P, Anjusha K (2019) Assessment of probiotic in aquaculture: functional changes and impact on fish gut Microbiol Res J Int 1–10. https://doi.org/10.9734/mrji/2019/v29i130156
Parker R (1974) Probiotics, the other half of the antibiotic story. Anim Nutr Health 29:4–8. https://ci.nii.ac.jp/naid/10014898669/#cit
Dawood MA, Koshio S, Ishikawa M, Yokoyama S, El Basuini MF, Hossain MS, Nhu TH, Dossou S, Moss AS (2016) Effects of dietary supplementation of Lactobacillus rhamnosus or/and Lactococcus lactis on the growth, gut microbiota and immune responses of red sea bream, Pagrus major. Fish Shellfish Immunol 49:275–285. https://doi.org/10.1016/j.fsi.2015.12.047
Hoseinifar SH, Sun Y-Z, Wang A, Zhou Z (2018) Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front Microbiol 9:2429. https://doi.org/10.3389/fmicb.2018.02429
Ringø E, Hoseinifar SH, Ghosh K, Doan HV, Beck BR, Song SK (2018) Lactic acid bacteria in finfish—an update. Front Microbiol 9:1818. https://doi.org/10.3389/fmicb.2018.01818
Gatesoupe F-J (2008) Updating the importance of lactic acid bacteria in fish farming: natural occurrence and probiotic treatments. J Mol Microbiol Biotechnol 14:107–114. https://doi.org/10.1159/000106089
Lara-Flores M (2011) The use of probiotic in aquaculture: an overview. Int Res J Microbiol 2:471–478. https://www.researchgate.net/profile/Maurilio_Lara-flores/publication/230807485_The_use_of_probiotic_in_aquaculture_an_overview/links/0912f504a0a93e7b71000000.pdf
Qin C, Xu L, Yang Y, He S, Dai Y, Zhao H, Zhou Z (2014) Comparison of fecundity and offspring immunity in zebrafish fed Lactobacillus rhamnosus CICC 6141 and Lactobacillus casei BL23. Reprod 147:53–64. https://doi.org/10.1530/rep-13-0141
Hoseinifar SH, Sun YZ, Caipang CM (2017) Short-chain fatty acids as feed supplements for sustainable aquaculture: an updated view. Aquac Res 48:1380–1391. https://doi.org/10.1111/are.13239
Farsani MN, Gorji SB, Hoseinifar SH, Rashidian G, Van Doan H (2020) Combined and singular effects of dietary PrimaLac® and potassium diformate (KDF) on growth performance and some physiological parameters of rainbow trout (Oncorhynchus mykiss). Probiotics Antimicrob Proteins 12:236–245. https://doi.org/10.1007/s12602-019-9523-2
Akbari Nargesi E, Falahatkar B, Sajjadi MM (2020) Dietary supplementation of probiotics and influence on feed efficiency, growth parameters and reproductive performance in female rainbow trout (Oncorhynchus mykiss) broodstock. Aquac Nutr 26:98–108. https://doi.org/10.1111/anu.12970
Akbari Nargesi E, Falahatkar B, Mohammadi M (2019) Growth performance and hematological indices in rainbow trout (Oncorhynchus mykiss): exclusive study of probiotic effect on male broodstock. Iran Fish Sci J 20(5):1304–1316. https://doi.org/10.22092/ISFJ.2019.119
Ramzannejad O, Changizi R, Vatandoust S, Safari R, Manouchehri H (2021) The effect of probiotic Bio-Aqua® on growth performance, haematological and biochemical parameters of bighead carp (Hypophthalmichthys nobilis). Iran J Fish Sci 20(5): 1304–1316. http://jifro.ir/article-1-4365-en.html
Mohammadian T, Alishahi M, Tabandeh MR, Ghorbanpoor M, Gharibi D (2018) Changes in immunity, expression of some immune-related genes of Shabot fish, Tor grypus, following experimental infection with Aeromonas hydrophila: effects of autochthonous probiotics. Probiotics Antimicrob Proteins 10:616–628. https://doi.org/10.1007/s12602-017-9373-8
Bai S, Katya K, Yun H (2015) Additives in aquafeed. Feed and Feeding Practices in Aquaculture. https://doi.org/10.1016/B978-0-08-100506-4.00007-6
Elala NMA, Ragaa NM (2015) Eubiotic effect of a dietary acidifier (potassium diformate) on the health status of cultured Oreochromis niloticus. J Adv Res 6:621–629. https://doi.org/10.1016/j.jare.2014.02.008
Ng WK, Koh CB (2017) The utilization and mode of action of organic acids in the feeds of cultured aquatic animals. Rev Aquac 9:342–368. https://doi.org/10.1111/raq.12141
Kovanda L, Zhang W, Wei X, Luo J, Wu X, Atwill ER, Vaessen S, Li X, Liu Y (2019) In vitro antimicrobial activities of organic acids and their derivatives on several species of gram-negative and gram-positive bacteria. Molecules 24:3770. https://doi.org/10.3390/molecules24203770
Tran NT, Li Z, Wang S, Zheng H, Aweya JJ, Wen X, Li S (2020) Progress and perspectives of short-chain fatty acids in aquaculture. Rev Aquac 12:283–298. https://doi.org/10.1111/raq.12317
Sangari M, Sotoudeh E, Bagheri D, Morammazi S, Mozanzadeh MT (2021) Growth, body composition, and hematology of yellowfin seabream (Acanthopagrus latus) given feeds supplemented with organic acid salts (sodium acetate and sodium propionate). Aquac Int 29:261–273. https://doi.org/10.1007/s10499-020-00625-x
Castillo S, Rosales M, Pohlenz C, Gatlin DM III (2014) Effects of organic acids on growth performance and digestive enzyme activities of juvenile red drum Sciaenops ocellatus. Aquaculture 433:6–12. https://doi.org/10.1016/j.aquaculture.2014.05.038
Morken T, Kraugerud OF, Barrows FT, Sørensen M, Storebakken T, Øverland M (2011) Sodium diformate and extrusion temperature affect nutrient digestibility and physical quality of diets with fish meal and barley protein concentrate for rainbow trout (Oncorhynchus mykiss). Aquaculture 317:138–145. https://doi.org/10.1016/j.aquaculture.2011.04.020
Jedi Mostafaloo A, Hedayatifard M, Keshavarz M, Mohammadian T (2021) Effects of different levels of sodium diformate and formic acid salt on growth performance, digestive enzymes, and innate immunological parameters of beluga (Huso huso) juveniles. Iran J Fish Sci 20(3):879–900. https://doi.org/10.22092/ijfs.2021.350956.0
Vielma J, Lall S (1997) Dietary formic acid enhances apparent digestibility of minerals in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Nutr 3:265–268. https://doi.org/10.1111/j.1365-2095.1997.00041.x
Davies S, Guroy D, Hassaan MS, El-Ajnaf S, El-Haroun E (2020) Evaluation of co-fermented apple-pomace, molasses and formic acid generated sardine based fish silages as fishmeal substitutes in diets for juvenile European sea bass (Dicentrachus labrax) production. Aquaculture 521:735087. https://doi.org/10.1016/j.aquaculture.2020.735087
Hassaan MS, El-Sayed A, Mohammady EY, Zaki MA, Elkhyat MM, Jarmołowicz S, El-Haroun ER (2021) Eubiotic effect of a dietary potassium diformate (KDF) and probiotic (Lactobacillus acidophilus) on growth, hemato-biochemical indices, antioxidant status and intestinal functional topography of cultured Nile tilapia Oreochromis niloticus fed diet free fishmeal. Aquaculture 533:736147. https://doi.org/10.1016/j.aquaculture.2020.736147
Siqwepu O, Salie K, Goosen N (2020) Evaluation of potassium diformate and potassium chloride in the diet of the African catfish, Clarias gariepinus in a recirculating aquaculture system. Aquaculture 526:735414. https://doi.org/10.1016/j.aquaculture.2020.735414
Suphoronski S, Chideroli R, Facimoto C, Mainardi R, Souza F, Lopera-Barrero N, Jesus G, Martins M, Di Santis G, De Oliveira A (2019) Effects of a phytogenic, alone and associated with potassium diformate, on tilapia growth, immunity, gut microbiome and resistance against francisellosis. Sci Rep 9:1–14. https://doi.org/10.1038/s41598-019-42480-8
Wassef EA, Abdel-Momen SA-G, Saleh NE-S, Al-Zayat AM, Ashry AM (2017) Is sodium diformate a beneficial feed supplement for European seabass (Dicentrarchus labrax)? Effect on growth performance and health status. Egypt J Aquat Res 43:229–234. https://doi.org/10.1016/j.ejar.2017.09.005
Ng WK, Koh CB, Sudesh K, Siti-Zahrah A (2009) Effects of dietary organic acids on growth, nutrient digestibility and gut microflora of red hybrid tilapia, Oreochromis sp., and subsequent survival during a challenge test with Streptococcus agalactiae. Aquac Res 40:1490–1500. https://doi.org/10.1111/j.1365-2109.2009.02249.x
Yustiati A, Nadiyah NA, Suryadi IBB, Rosidah R (2019) Immune performances of sangkuriang catfish (Clarias gariepinus) with addition of potassium diformate on feed. World News Nat Sciences 25:113–129. http://psjd.icm.edu.pl/psjd/element/bwmeta1.element.psjd-acda1328-ad8c-4725-99bf-f972f9cef822
Kalantarian S, Mirzargar S, Rahmati-Holasoo H, Sadeghinezhad J, Mohammadian T (2020) Effects of oral administration of acidifier and probiotic on growth performance, digestive enzymes activities and intestinal histomorphology in Salmo trutta caspius (Kessler, 1877). Iran J Fish Sci 19:1532–1555. https://doi.org/10.22092/ijfs.2019.119077
Mohammadi MJ (2020) Effects of sodium diformate and citric acide on growth performance, immune and hematological parameters of the juvenile Oncorhynchus mykiss. ISFJ. 29:117–129. http://isfj.ir/article-1-2188-en.html
Dostani Dezfoli M, Rajabzadeh Ghatrami E, Abdi R (2020) Effects of sodium diformate and citric acid on body composition, serum enzymes activity and alimentary canal tissue of the juvenile rainbow trout (Oncorhynchus mykiss). J Aqua Ani Nutri 5:27–38. https://janb.guilan.ac.ir/article_4112.html?lang=en
Reyshari A, Mohammadiazarm H, Mohammadian T, Torfi Mozanzadeh M (2019) Effects of sodium diformate on growth performance, gut microflora, digestive enzymes and innate immunological parameters of Asian sea bass (Lates calcarifer) juveniles. Aquac Nutr 25:1135–1144. https://doi.org/10.1111/anu.12929
Ebrahimi M, Daeman NH, Chong CM, Karami A, Kumar V, Hoseinifar SH, Romano N (2017) Comparing the effects of different dietary organic acids on the growth, intestinal short-chain fatty acids, and liver histopathology of red hybrid tilapia (Oreochromis sp.) and potential use of these as preservatives. Fish Physiol Biochem 43:1195–1207. https://doi.org/10.1007/s10695-017-0365-0
Tran-Ngoc KT, Huynh ST, Sendão J, Nguyen TH, Roem AJ, Verreth JA, Schrama JW (2019) Environmental conditions alter the effect of organic acid salts on digestibility and intestinal morphology in Nile tilapia (Oreochromis niloticus). Aquac Nutr 25:134–144. https://doi.org/10.1111/anu.12837
Krome C, Schuele F, Jauncey K, Focken U (2018) Influence of a sodium formate/formic acid mixture on growth of juvenile common carp (Cyprinus carpio) fed different fishmeal replacement levels of detoxified Jatropha curcas kernel meal in practical, mixed diets. J Appl Aquac 30:137–156. https://doi.org/10.1080/10454438.2017.1412845
Maktabi P, Mohammadiazarm H, Peyghan R, Mousavi M, Zarei M (2019) Effect of formic acid, potassium Di format and formic acid nano-chitosan solution on survival, growth indices and body composition of common carp (Cyprinus carpio).J Wetland Ecobio 10:41–54. http://jweb.iauahvaz.ac.ir/article-1-729-en.html
Heshmatfar F, Shabany A, Hoseinifar H, Ghafari H (2020) Effects of singular or combined administration of formic acid and Pediococcus acidilactici on growth indices and resistance to salinity in common carp fingerling (Cyprinus carpio). Utilizati Cultiv Aqua 9:83–91. https://doi.org/10.22069/JAPU.2020.16741.1506
Mohammadian T, Dezfuly ZT, Motlagh RG, Jangaran-Nejad A, Hosseini SS, Khaj H, Alijani N (2019) Effect of encapsulated lactobacillus bulgaricus on innate immune system and hematological parameters in rainbow trout (Oncorhynchus mykiss), post-administration of Pb. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-019-09544-7
Sharifuzzaman S, Austin B (2009) Influence of probiotic feeding duration on disease resistance and immune parameters in rainbow trout. Fish Shellfish Immunol 27:440–445. https://doi.org/10.1016/j.fsi.2009.06.010
Quade MJ, Roth JA (1997) A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet immunol immunop 58:239–248. https://doi.org/10.1016/S0165-2427(97)00048-2
Barta O (1993) Immunologic techniques evaluating cells and their functions. Veterinary Clinical Immunology Laboratory Bar-Lab. Inc, USA B 1:1-B3
Mohammadian T, Alishahi M, Tabandeh MR, Ghorbanpoor M, Gharibi D, Tollabi M, Rohanizade S (2016) Probiotic effects of Lactobacillus plantarum and L. delbrueckii ssp. bulguricus on some immune-related parameters in Barbus grypus. Aquac Int 24:225–242. https://doi.org/10.1007/s10499-015-9921-8
Kurhaluk N, Bojkova B, Radkowski M, Zaitseva OV, Kyriienko S, Demkow U, Winklewski PJ (2017) Melatonin and metformin diminish oxidative stress in heart tissue in a rat model of high fat diet and mammary carcinogenesis. Clin Invest. Springer, pp 7–19. https://doi.org/10.1007/5584_2017_128
Peixoto FP, Carrola J, Coimbra AM, Fernandes C, Teixeira P, Coelho L, Conceição I, Oliveira MM, Fontainhas-Fernandes A (2013) Oxidative stress responses and histological hepatic alterations in barbel, Barbus bocagei, from Vizela River, Portugal. Revista Internacional de Contaminacion Ambiental 291:29–38. https://www.redalyc.org/pdf/370/37025634003.pdf
Ghanei-Motlagh R, Gharibi D, Mohammadian T, Khosravi M, Mahmoudi E, Zarea M, El-Matbouli M, Menanteau-Ledouble S (2021) Feed supplementation with quorum quenching probiotics with anti-virulence potential improved innate immune responses, antioxidant capacity and disease resistance in Asian seabass (Lates calcarifer). Aquaculture. https://doi.org/10.1016/j.aquaculture.2021.736345
Mohammadian T, Nasirpour M, Tabandeh MR, Heidary AA, Ghanei-Motlagh R, Hosseini SS (2019) Administrations of autochthonous probiotics altered juvenile rainbow trout Oncorhynchus mykiss health status, growth performance and resistance to Lactococcus garvieae, an experimental infection. Fish Shellfish Immunol 86:269–279. https://doi.org/10.1016/j.fsi.2018.11.052
Mohammadian T, Nasirpour M, Tabandeh MR, Mesbah M (2019) Synbiotic effects of β-glucan, mannan oligosaccharide and Lactobacillus casei on growth performance, intestine enzymes activities, immune-hematological parameters and immune-related gene expression in common carp, Cyprinus carpio: an experimental infection with Aeromonas hydrophila. Aquaculture 511:634197. https://doi.org/10.1016/j.aquaculture.2019.06.011
Zare R, Kenari AA, Sadati MY (2021) Influence of dietary acetic acid, protexin (probiotic), and their combination on growth performance, intestinal microbiota, digestive enzymes, immunological parameters, and fatty acids composition in Siberian sturgeon (Acipenser baerii, Brandt, 1869). Aquacult Int 29(3):891–910. https://doi.org/10.1007/s10499-021-00652-2
Chauhan A, Singh R (2019) Probiotics in aquaculture: a promising emerging alternative approach. Symbiosis 77:99–113. https://doi.org/10.1007/s13199-018-0580-1
Sayes C, Leyton Y, Riquelme C (2018) Probiotic bacteria as an healthy alternative for fish aquaculture. Antibiotics use in animals, Savic, S, editor Rijeka, Croatia: InTech Publishers:115–132
Fuchs V, Schmidt J, Slater M, Zentek J, Buck B, Steinhagen D (2015) The effect of supplementation with polysaccharides, nucleotides, acidifiers and Bacillus strains in fish meal and soy bean based diets on growth performance in juvenile turbot (Scophthalmus maximus). Aquaculture 437:243–251. https://doi.org/10.1016/j.aquaculture.2014.12.007
Tabrizi JM, Barzeghar A, Farzampour S, Mirzaii H, Safarmashaei S (2012) Study of the effect of prebiotic (Saccharomyces cerevisiae) and acidifier on growth parameters in grower’s rainbow trout (Oncorhynchus mykiss). Annals of Biol Res 3:2053–2057. http://scholarsresearchlibrary.com/ABR-vol3-iss5/ABR-2012-3-5-2053-2057.pdf
Ganguly S, Dora KC, Sarkar S, Chowdhury S (2013) Supplementation of prebiotics in fish feed: a review. Rev Fish Biol Fish 23(2):195–199. https://doi.org/10.1007/s11160-012-9291-5
Ringø E, Løvmo L, Kristiansen M, Bakken Y, Salinas I, Myklebust R, Olsen RE, Mayhew TM (2010) Lactic acid bacteria vs. pathogens in the gastrointestinal tract of fish: a review. Aquac Res 41:451–467. https://doi.org/10.1111/j.1365-2109.2009.02339.x
Ringø E, Bendiksen H, Wesmajervi M, Olsen R, Jansen P, Mikkelsen H (2000) Lactic acid bacteria associated with the digestive tract of Atlantic salmon (Salmo salar L.). J Appl Microbiol 89:317–322. https://doi.org/10.1046/j.1365-2672.2000.01116.x
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29(1):2–14. https://doi.org/10.1016/j.fsi.2010.02.017
Lindsay GJ (1986) The significance of chitinolytic enzymes and lysozyme in rainbow trout (Salmo gairdneri) defence. Aquaculture 51(3–4):169–173. https://doi.org/10.1016/0044-8486(86)90306-6
Nikoskelainen S, Ouwehand AC, Bylund G, Salminen S, Lilius E-M (2003) Immune enhancement in rainbow trout (Oncorhynchus mykiss) by potential probiotic bacteria (Lactobacillus rhamnosus). Fish Shellfish Immunol 15:443–452. https://doi.org/10.1016/S1050-4648(03)00023-8
Korkea-Aho T, Papadopoulou A, Heikkinen J, Von Wright A, Adams A, Austin B, Thompson K (2012) Pseudomonas M162 confers protection against rainbow trout fry syndrome by stimulating immunity. J Appl Microbiol 113:24–35. https://doi.org/10.1111/j.1365-2672.2012.05325.x
Jami MJ, Kenari AA, Paknejad H, Mohseni M (2019) Effects of dietary b-glucan, mannan oligosaccharide, Lactobacillus plantarum and their combinations on growth performance, immunity and immune related gene expression of Caspian trout, Salmo trutta caspius (Kessler, 1877). Fish Shellfish Immunol 91:202–208. https://doi.org/10.1016/j.fsi.2019.05.024
Ahmadifar E, Sadegh TH, Dawood MA, Dadar M, Sheikhzadeh N (2020) The effects of dietary Pediococcus pentosaceus on growth performance, hemato-immunological parameters and digestive enzyme activities of common carp (Cyprinus carpio). Aquaculture 516:734656. https://doi.org/10.1016/j.aquaculture.2019.734656
Lim C, Klesius PH, Luckstadt C (2010) Effects of dietary levels of potassium diformate on growth, feed utilization and resistance to Streptococcus iniae of Nile tilapia, Oreochromis niloticus. In 14th Int Sympo Fish Nutri Feed. Qingdao, China (p. 472)
Andani H, Tukmechi A, Meshkini S, Sheikhzadeh N (2012) Antagonistic activity of two potential probiotic bacteria from fish intestines and investigation of their effects on growth performance and immune response in rainbow trout (Oncorhynchus mykiss). J Appl Ichthyol 28:728–734. https://doi.org/10.1111/j.1439-0426.2012.01974.x
Sun YZ, Yang HL, Ma RL, Song K, Li JS (2012) Effect of Lactococcus lactis and Enterococcus faecium on growth performance, digestive enzymes and immune response of grouper Epinephelus coioides. Aquac Nutr 18(3):281–289. https://doi.org/10.1111/j.1365-2095.2011.00894.x
Mehrim AI (2014) Physiological, biochemical and histometric responses of Nile tilapia (Oreochromis niloticus L.) by dietary organic chromium (chromium picolinate) supplementation. J Adv Res 5:303–310. https://doi.org/10.1016/j.jare.2013.04.002
Vazirzadeh A, Roosta H, Masoumi H, Farhadi A, Jeffs A (2020) Long-term effects of three probiotics, singular or combined, on serum innate immune parameters and expressions of cytokine genes in rainbow trout during grow-out. Fish Shellfish Immunol 98:748–757. https://doi.org/10.1016/j.fsi.2019.11.023
Tachibana L, Telli GS, de Carla DD, Gonçalves GS, Ishikawa CM, Cavalcante RB, Natori MM, Hamed SB, Ranzani-Paiva MJT (2020) Effect of feeding strategy of probiotic Enterococcus faecium on growth performance, hematologic, biochemical parameters and non-specific immune response of Nile tilapia. Aquac Rep 16:100277. https://doi.org/10.1016/j.aqrep.2020.100277
Aftabgard M, Salarzadeh A, Mohseni M, Shabanipour AHB, Zorriehzahra MEJ (2019) The combined efficiency of dietary isomaltooligosaccharides and Bacillus spp. on the growth, hemato-serological, and intestinal microbiota indices of caspian brown trout (Salmo trutta caspius Kessler, 1877). Probiotics Antimicrob Proteins 11:198–206. https://doi.org/10.1007/s12602-017-9361-z
Dalmo R, Ingebrigtsen K, Bøgwald J (1997) Non-specific defence mechanisms in fish, with particular reference to the reticuloendothelial system (RES). J Fish Dis 20:241–273. https://doi.org/10.1046/j.1365-2761.1997.00302.x
Wassef EA, Saleh NE, Abdel-Meguid NE, Barakat KM, Abdel-Mohsen HH, El-bermawy NM (2020) Sodium propionate as a dietary acidifier for European seabass (Dicentrarchus labrax) fry: immune competence, gut microbiome, and intestinal histology benefits. Aquac Int 28:95–111. https://doi.org/10.1007/s10499-019-00446-7
Newaj-Fyzul A, Adesiyun AA, Mutani A, Ramsubhag A, Brunt J, Austin B (2007) Bacillus subtilis AB1 controls Aeromonas infection in rainbow trout (Oncorhynchus mykiss, Walbaum). J Appl Microbiol 103:1699–1706. https://doi.org/10.1111/j.1365-2672.2007.03402.x
Gobi N, Vaseeharan B, Chen J-C, Rekha R, Vijayakumar S, Anjugam M, Iswarya A (2018) Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol 74:501–508. https://doi.org/10.1016/j.fsi.2017.12.066
Abdel-Tawwab M, Ahmad MH, Khattab YA, Shalaby AM (2010) Effect of dietary protein level, initial body weight, and their interaction on the growth, feed utilization, and physiological alterations of Nile tilapia, Oreochromis niloticus (L.). Aquaculture 298:267–274. https://doi.org/10.1016/j.aquaculture.2009.10.027
Imanpoor M, Roohi Z (2015) Effect of a multi-strain probiotic (Primalac) on growth performance, some blood biochemical parameters, survival and stress resistance on Caspian kutum (Rutilus kutum) fry. Iran Fish Sci J 24:95–102. http://aquaticcommons.org/id/eprint/22056
Nafisi Bahabadi M, Ch A (2015) Effects of dietary probiotic (Lactobacillus plantarum) on body composition, serum biochemical parameters and liver enzymes of Asian sea bass (Lates calcalifer, Bloch 1790). J Mar Sci Technol 14(2), 1–14. https://doi.org/10.22113/jmst.2015.8579
Panigrahi A, Kiron V, Satoh S, Watanabe T (2010) Probiotic bacteria Lactobacillus rhamnosus influences the blood profile in rainbow trout Oncorhynchus mykiss (Walbaum). Fish Physiol Biochem 36(4):969–977. https://doi.org/10.1007/s10695-009-9375-x
Yang S-P, Wu Z-H, Jian J-C, Zhang X-Z (2010) Effect of marine red yeast Rhodosporidium paludigenum on growth and antioxidant competence of Litopenaeus vannamei. Aquaculture 309:62–65. https://doi.org/10.1016/j.aquaculture.2010.09.032
Hoseinifar SH, Yousefi S, Van Doan H, Ashouri G, Gioacchini G, Maradonna F, Carnevali O (2020) Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. Rev Fish Sci Aquac. https://doi.org/10.1080/23308249.2020.1795616
Guzmán-Villanueva LT, Ascencio-Valle F, Macías-Rodríguez ME, Tovar-Ramírez D (2014) Effects of dietary β-1, 3/1, 6-glucan on the antioxidant and digestive enzyme activities of Pacific red snapper (Lutjanus peru) after exposure to lipopolysaccharides. Fish Physiol Biochem 40:827–837. https://doi.org/10.1007/s10695-013-9889-0
Zhang C-N, Li X-F, Xu W-N, Jiang G-Z, Lu K-L, Wang L-N, Liu W-B (2013) Combined effects of dietary fructooligosaccharide and Bacillus licheniformis on innate immunity, antioxidant capability and disease resistance of triangular bream (Megalobrama terminalis). Fish Shellfish Immunol 35:1380–1386. https://doi.org/10.1016/j.fsi.2013.07.047
Hoseinifar SH, Hoseini SM, Bagheri D (2017) Effects of galactooligosaccharide and Pediococcus acidilactici on antioxidant defence and disease resistance of rainbow trout, Oncorhynchus mykiss. Ann Anim Scie 17:217–227. https://doi.org/10.1515/aoas-2016-0024
Del Rio D, Stewart AJ, Pellegrini N (2005) A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis 15:316–328. https://doi.org/10.1016/j.numecd.2005.05.003
Shen WY, Fu LL, Li WF, Zhu YR (2010) Effect of dietary supplementation with Bacillus subtilis on the growth, performance, immune response and antioxidant activities of the shrimp (Litopenaeus vannamei). Aquac Res 41:1691–1698. https://doi.org/10.1111/j.1365-2109.2010.02554.x
Esteban MA, Cordero H, Martínez-Tomé M, Jiménez-Monreal AM, Bakhrouf A, Mahdhi, A (2014) Effect of dietary supplementation of probiotics and palm fruits extracts on the antioxidant enzyme gene expression in the mucosae of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 39(2), 532–540. https://doi.org/10.1016/j.fsi.2014.06.012
Jinendiran S, Boopathi S, Sivakumar N, Selvakumar G (2019) Functional characterization of probiotic potential of novel pigmented bacterial strains for aquaculture applications. Probiotics Antimicrob Proteins 11(1):186–197. https://doi.org/10.1007/s12602-017-9353-z
Pérez-Sánchez T, Balcázar JL, Merrifield DL, Carnevali O, Gioacchini G, de Blas I, Ruiz-Zarzuela I (2011) Expression of immune-related genes in rainbow trout (Oncorhynchus mykiss) induced by probiotic bacteria during Lactococcus garvieae infection. Fish Shellfish Immunol 31:196–201. https://doi.org/10.1016/j.fsi.2011.05.005
Feng J, Chang X, Zhang Y, Yan X, Zhang J, Nie G (2019) Effects of Lactococcus lactis from Cyprinus carpio L. as probiotics on growth performance, innate immune response and disease resistance against Aeromonas hydrophila. Fish Shellfish Immunol 93:73–81. https://doi.org/10.1016/j.fsi.2019.07.028
Reda RM, Mahmoud R, Selim KM, El-Araby IE (2016) Effects of dietary acidifiers on growth, hematology, immune response and disease resistance of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 50:255–262. https://doi.org/10.1016/j.fsi.2016.01.040
Selim KM, Reda RM, Mahmoud R, El-Araby IE (2020) Effects of nucleotides supplemented diets on growth performance and expressions of ghrelin and insulin-like growth factor genes in Nile tilapia, Oreochromis niloticus. J Appl Aquac 32:157–174. https://doi.org/10.1080/10454438.2019.1696911
Midhun SJ, Neethu S, Arun D, Vysakh A, Divya L, Radhakrishnan E, Jyothis M (2019) Dietary supplementation of Bacillus licheniformis HGA8B improves growth parameters, enzymatic profile and gene expression of Oreochromis niloticus. Aquaculture 505:289–296. https://doi.org/10.1016/j.aquaculture.2019.02.064
Funding
A grant from Shahid Chamran University of Ahvaz Research Council funded this work (Grant No: 26247, 1397.3.2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammadian, T., Momeni, H., kazemi, M. et al. Eubiotic Effect of a Dietary Bio-Aqua® and Sodium Diformate (NaDF) on Salmo trutta caspius: Innate Immune System, Biochemical Indices, Antioxidant Defense, and Expression of Immunological and Growth-Related Genes. Probiotics & Antimicro. Prot. 15, 1342–1354 (2023). https://doi.org/10.1007/s12602-022-09965-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-09965-x