Abstract
Obesity is one of the chronic diseases that increase annually and cause cardiovascular disease, which is the main cause of death worldwide. So, this study aims to evaluate the role of the two potential probiotics: Lactiplantibacillus plantarum Pro1 and Lacticaseibacillus rhamnosus Pro2, isolated from the fermented milk and corn silage as anti-obesity supplements. Seventy-five male BALB/c mice were distributed to five groups (control, obesity, obesity plus L. plantarum (OLP), obesity plus L. rhamnosus (OLR) and obesity plus mixture of two strains (OM)). The body weight, lipid profile, histopathology and enzymes of liver were assessed. RT-PCR was used to determine the expression of CYP7A1, ALTG4, TNFα and ROR genes.
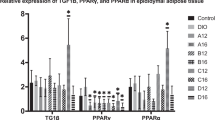
The findings show that the obesity group recorded the significant highest value of the body weight, TC, TG, LDL, AST and ALT, while OLP group recorded the significant lowest value. Liver tissue of obesity group has necrosis and fatty changes, while the OLP group was related to the control group. The findings of RT-PCR show non-significant differences between the control group and the OLP group, with significant differences between the control group and the set groups in expression of CYP7A1, ALTG4, TNFα and ROR genes. L. plantarum Pro1 reduced the expression of inflammation genes (TNFα and ROR), and increase the expression of the lipid metabolism genes (CYP7A1, ALTG4) to reduce the inflammatory effects of obesity in the liver, and decrease the cholesterol level in serum. Therefore, L. plantarum Pro1 is useful as anti-obesity supplements and an eliminator of the relevant diseases.




Similar content being viewed by others
Data Availability
Data and material are available for all.
References
World Health Organization (WHO) (2009) Cardiovascular disease; Fact. Sheet n_317; WHO: Geneva, Swizerland
DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittmann BE (2008) Gut microbiota and its possible relationship with obesity. Mayo Clin Proc 83(4):460–469. https://doi.org/10.4065/83.4.460
Fradi I, Drissa MA, Cheour M, Meddeb I, Drissa H (2008) Coronary atherosclerosis and familial hypercholesterolemia: A case report. Tunis Med 86(2):200–202
Bliznakov EG (2002) Lipid-lowering drugs (statins), cholesterol, and coenzyme Q10. The Baycol case: A modern Pandora’s Box. Biomed Pharmacother 56(1): 56–59. https://doi.org/10.1016/S0753-3322(01)00150-0
Fuller R (1992) History and development of probiotics. Pages 1–8 in Probiotics—The Scientific Basis. R. Fuller, ed. Chapman and Hall, London, UK. https://doi.org/10.1007/978-94-011-2364-8_1
Pavli F, Tassou C, Nychas GJ, Chorianopoulos N (2018) Probiotic incorporation in edible films and coatings: bioactive solution for functional foods. Int J Mol Sci 19(1):150. https://doi.org/10.3390/ijms19010150
Wu CH, Hsueh YH, Kuo JM, Liu SJ (2018) Characterization of a potential probiotic Lactobacillus brevis RK03 and efficient production of -aminobutyric acid in batch fermentation. Int J Mol Sci 19(1):143. https://doi.org/10.3390/ijms19010143
Wang Y, Xu N, Xi A, Ahmed Z, Zhang B, Bai XE (2009) Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl Microbiol Biotechnol 84(2):341–347. https://doi.org/10.1007/s00253-009-2012-x
Ding WR, Shi C, Chen M, Zhou JW, Long RJ, Guo XS (2017) Screening for lactic acid bacteria in traditional fermented Tibetan yak milk and evaluating their probiotic and cholesterol-lowering potentials in rats fed a high-cholesterol diet. J Funct Foods 32:324–332. https://doi.org/10.1016/j.jff.2017.03.021
Fuentes MC, Lajo T, Carrioon JM, Cune J (2013) Cholesterol-lowering efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. Br J Nutr 109(10):1866–1872. https://doi.org/10.1017/S000711451200373X
Jones ML, Martoni CJ, Parent M, Prakash S (2012) Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br J Nutr 107(10):1505–1513. https://doi.org/10.1017/S0007114511004703
Changlu M, Shuwen Z, Jing L, Cai Z, Xiaoyang P, Jiaping LV (2019) Screening for cholesterol-lowering probiotics from lactic acid bacteria isolated from corn silage based on three hypothesized pathways. Int J Mol Sci 20(9):2073. https://doi.org/10.3390/ijms20092073
Park JE, Oh SH, Cha YS (2014) Lactobacillus plantarum LG42 isolated from gajami sikhae decreases body and fat pad weights in diet-induced obese mice. J App Microbiol 116(1):145–156. https://doi.org/10.1111/jam.12354
Tiangang L, Erika O, Michelle M, Peter H, Colleen MN, John YLC (2010) Transgenic expression of cholesterol7α-hydroxylase in the liver prevents high-fat diet–induced obesity and insulin resistance in mice. Hepatology 52(2):678–690. https://doi.org/10.1002/hep.23721
Anne SH, Kristy AA, Amanda MD, Mark HK (2011) A chronic high-cholesterol diet paradoxically suppresses hepatic CYP7A1 expression in FVB/NJ mice. J Lipid Res 52(2):289–298. https://doi.org/10.1194/jlr.M012781
Bailetti D, Bertoccini L, Mancina RM, Barchetta I et al (2018) ANGPTL4 gene E40K variation protects against obesity-associated dyslipidemia in participants with obesity. Obes Sci Pract 5(1):83–90. https://doi.org/10.1002/osp4.311
Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ et al (2008) Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 48(2):474–486. https://doi.org/10.1002/hep.22363
Wang B, Wang Z, Li N, Li Y, Li Q, Li J (2010) The isolation of Lactobacillus strains from human gut for use as potential probiotics. Int J Probiotics Prebiotics 5:97–104
Khattab AA (2002) Molecular and biochemical studies of genetically constructed lactic acid bacteria. Tanta Univ., Faculty of Agriculture, Egypt, PhD, Genetics Dept
Yoo SR, Kim YJ, Park DY, Jung UJ et al (2013) Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity 21(12):2571–2578. https://doi.org/10.1002/oby.20428
Park DY, Ahn YT, Park SH, Huh CS et al (2013) Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One 8:e59470. https://doi.org/10.1371/journal.pone.0059470
Wang J, Tang H, Zhang C, Zhao Y et al (2015) Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J 9:1–15. https://doi.org/10.1038/ismej.2014.99
Kiessling G, Schneider J, Jahreis G (2002) Long-term consumption of fermented dairy products over 6 months increases HDL cholesterol. Eur J Clin Nutr 56(9):843–849. https://doi.org/10.1038/sj.ejcn.1601399
Ito M, Kusuhara S, Yokoi W, Sato T et al (2017) Streptococcus thermophiles fermented milk reduces serum MDA-LDL and blood pressure in healthy and mildly hypercholesterolaemic adults. J Benef Microbes 8(2):171–178. https://doi.org/10.3920/BM2016.0102
Zhang F, Qiu l, Xu X et al (2017) Beneficial effects of probiotic cholesterol-lowering strain on Enterococcus faecium WEFA23 from infants on diet-induced metabolic syndrome in rats. J Dairy Sci 100(3):1618–1628. https://doi.org/10.3168/jds.2016-11870
Nazarii K, Caterina C, Giovanni C, Andreana PH, Igor S et al (2016) Probiotics in prevention and treatment of obesity: a critical view. Nutr Metab 13–14. https://doi.org/10.1186/s12986-016-0067-0
Caesar R, Fåk F, Bäckhed F (2010) Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J Intern Med 268(4):320–328. https://doi.org/10.1111/j.1365-2796.2010.02270.x
De Clercq NC, Groen AK, Romijn JA, Nieuwdorp M (2016) Gut microbiota in obesity and undernutrition. Adv Nutr 7(6):1080–1089. https://doi.org/10.3945/an.116.012914
Tomás C, José AG, Mercedes GB, Cristina C (2019) The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 11(3):635. https://doi.org/10.3390/nu11030635
Reichold A, Brenner SA, Spruss A, Förster-Fromme K, Bergheim I, Bischoff SC (2014) Bifidobacterium adolescentis protects from the development of nonalcoholic steatohepatitis in a mouse model. J Nutr Biochem 25:118–125. https://doi.org/10.1016/j.jnutbio.2013.09.011
Yoon MY, Ekihiro S (2015) TNFα in liver fibrosis. Curr Pathobiol Rep 3(4):253–261. https://doi.org/10.1007/s40139-015-0093-z
Raichur S, Fitzsimmons RL, Myers SA, Pearen MA, Lau P (2010) Identification and validation of the pathways and functions regulated by the orphan nuclear receptor, ROR alpha1, in skeletal muscle. Nucleic Acids Res 38(13):4296–4312. https://doi.org/10.1093/nar/gkq180
Kang HS, Okamoto K, Takeda Y et al (2011) Transcriptional profiling reveals a role for ROR-α in regulating gene expression in obesity-associated inflammation and hepatic steatosis. Physiol Genomics 43(13):818–828. https://doi.org/10.1152/physiolgenomics.00206.2010
Olshan DS, Rader DJ (2018) Angiopoietin-like protein 4: a therapeutic target for triglycerides and coronary disease? J Clin Lipidol 12:583–587. https://doi.org/10.1016/j.jacl.2018.01.012
Davies BSJ (2018) Can targeting ANGPTL proteins improve glucose tolerance? Diabetologia 61(6):1277–1281. https://doi.org/10.1007/s00125-018-4604-4
Gusarova V (2018) Genetic inactivation of ANGPTL4 improves glucose homeostasis and is associated with reduced risk of diabetes. Nat Commun 13(9):2252. https://doi.org/10.1038/s41467-018-04611-z
Cinkajzlova A, Mraz M, Lacinova Z, Klouckova J et al (2018) Angiopoietin-like protein 3 and 4 in obesity, type 2 diabetes mellitus, and malnutrition: The effect of weight reduction and realimentation. Nutr Diabetes 8(1):21. https://doi.org/10.1038/s41387-018-0032-2
Yang LY, Yu CG, Wang XH, Yuan SS, Zhang LJ, Lang JN, Zhao D, Feng YM (2017) Angiopoietin-like protein 4 is a high-density lipoprotein (HDL) component for HDL metabolism and function in nondiabetic participants and type-2 diabetic patients. J Am Heart Assoc 6(6):e005973. https://doi.org/10.1161/JAHA.117.005973
Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ (1996) An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 383(6602):728–731. https://doi.org/10.1038/383728a0
Yingting D, Fan Z, Wenzhen Y, Yuhui W et al (2019) Hepatic cholesterol accumulation ascribed to the activation of ileum FxrFgf15 pathway inhibiting hepatic Cyp7a1 in high-fat diet-induced obesity rats. Life Sci 232:116638. https://doi.org/10.1016/j.lfs.2019.116638
Li T, Jahan A, Chiang JY (2006) Bile acids and cytokines inhibit the human cholesterol 7 alpha-hydroxylase gene via the JNK/c-jun pathway in human liver cells. Hepatology 43(6):1202–1206. https://doi.org/10.1002/hep.21183
Kim MS, Sweeney TR, Shigenaga JK, Chui LG, Moser A et al (2007) Tumor necrosis factor and interleukin 1 decrease RXRα, PPARα, PPARγ, LXRα, and the coactivators SRC-1, PGC-1α, and PGC-1β in liver cells. Metabolism 56(2):267–279. https://doi.org/10.1016/j.metabol.2006.10.007
Plaza-Diaz J, Gomez-Llorente C, Abadia-Molina F et al (2014) Effects of Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036 on hepatic steatosis in zucker rats. PLoS One 9(5)
Sun Y, Tang Y, Hou X, Wang H, Huang L, Wen J, Niu H, Zeng W et al (2020) Novel Lactobacillus reuteri HI120 affects lipid metabolism in C57BL/6 obese mice. Front Vet Sci 7:560241. https://doi.org/10.3389/fvets.2020.560241
Funding
No funding received.
Author information
Authors and Affiliations
Contributions
This study was done in collaboration with all authors. A.K. and A.D. designed this study. N.E., A.D. and A.K. participated in the conduct of the study. N.E. and A.D. analyzed the data. A.D. and A.K. drafted the manuscript. A.D. and N.E. critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The experimental procedure used in this investigation was approved (NRC: 2,020,107) by the Animal Care and Use Committee of National Research Centre in Egypt.
Consent for Publication
All authors have given consent for the paper to be published by the corresponding author.
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
ALSuhaymi, N., Darwish, A.M. & Khattab, A.EN. Assessment of Two Potential Probiotic Strains As Anti-Obesity Supplements Under High-Fat Feeding Conditions. Probiotics & Antimicro. Prot. 15, 856–867 (2023). https://doi.org/10.1007/s12602-022-09912-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-09912-w