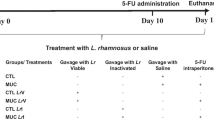
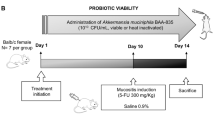
Abstract
Mucositis is one of the most strenuous side effects caused by chemotherapy drugs, such as 5-fluorouracil (5-FU), during the treatment of several types of cancers. The disease is so prevalent and aggressive that many patients cannot resist such symptoms. However, despite its frequency and clinical significance, there is no effective treatment to prevent or treat mucositis. Thus, the use of probiotics as an adjuvant for the treatment has gained prominence. In the present study, we evaluated the effectiveness of oral administration of the Antarctic strain of Rhodotorula mucilaginosa UFMGCB 18,377 as an alternative to minimize side effects of 5-FU-induced mucositis in mice. Body weight, food consumption, stool consistency, and presence of blood in the feces were assessed daily in mice orally treated or not with the yeast and submitted or not to experimental mucositis. Blood, bones, and intestinal tissues and fluid were used to determine intestinal permeability and immunological, microbiological, and histopathological parameters. Treatment with R. mucilaginosa UFMGCB 18,377 was able to decrease clinical signs of the disease, such as reduction of food intake and body weight loss, and also decreased the number of intestinal enterobacteria and intestinal length shortening. Additionally, treatment was able to decrease the levels of MPO and EPO activities and inflammatory infiltrates, as well as the histopathological lesions characteristic of mucositis in the jejunum and ileum. Results of the present study showed that the oral administration of R. mucilaginosa UFMGCB 18,377 protected mice against mucositis induced by 5-FU.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Longley DB, Harkin DP, Johnston PG (2003) 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 3:330–338. https://doi.org/10.1038/nrc1074
Sonis ST (1998) Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol 34:39–43. https://doi.org/10.1016/s1368-8375(97)00053-5
Harris DJ (2006) Cancer treatment-induced mucositis pain: strategies for assessment and management. Ther Clin Risk Manag 2:251–258. https://doi.org/10.2147/tcrm.2006.2.3.251
Lopes NN, Plapler H, Lalla RV, Chavantes MC, Yoshimura EM, Da Silva MA, Alves MT (2010) Effects of low-level laser therapy on collagen expression and neutrophil infiltrate in 5-fluorouracil-induced oral mucositis in hamsters. Lasers Surg Med 42:546–552. https://doi.org/10.1002/lsm.20920
Prisciandaro LD, Geier MS, Chua AE, Butler RN, Cummins AG, Sander GR, Howarth GS (2012) Probiotic factors partially prevent changes to caspases 3 and 7 activation and transepithelial electrical resistance in a model of 5-fluorouracil-induced epithelial cell damage. Support Care Cancer 20:3205–3210. https://doi.org/10.1007/s00520-012-1446-3
Sonis ST, Elting LS, Keefe D, Peterson M, Hauer-jensen M (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100:1995–2025. https://doi.org/10.1002/cncr.20162
Maioli TU, de Melo SB, Dias MN, Paiva NC, Cardoso VN, Fernandes SO, Carneiro CM, Martins SF, Generoso S (2014) Pretreatment with Saccharomyces boulardii does not prevent the experimental mucositis in Swiss mice. J Negat Results Biomed 13:1–8. https://doi.org/10.1186/1477-5751-13-6
Pokrowiecki R, Mielczarek A, Zare BAT, Tyski S (2017) Oral microbiome and periimplant diseases: where are we now? Ther Clin Risk Manag 13:1529–1542. https://doi.org/10.2147/TCRM.S139795
Yeoh ASJ, Gibson RJ, Yeoh EEK (2007) A novel animal model to investigate fractionated radiotherapy-induced alimentary mucositis: the role of apoptosis, p53, nuclear factor-kB, COX-1, and COX-2. Mol Cancer Ther 6:2319–2327. https://doi.org/10.1158/1535-7163.MCT-07-0113
Mccullough RW (2017) US oncology-wide incidence, duration, costs and deaths from chemoradiation mucositis and antimucositis therapy benefits. Future Oncol 13:2823–2852. https://doi.org/10.2217/fon-2017-0418
Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley DVS (2014) Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis. Current evidence and potential clinical applications. Aliment Pharmacol Ther 40:409–421. https://doi.org/10.1111/apt.12878
Gerhard D, Sousa F, Andraus R (2017) Probiotic therapy reduces inflammation and improves intestinal morphology in rats with induced oral mucositis. Braz Oral Res 31:e71. https://doi.org/10.1590/1807-3107BOR-2017.vol31.0071
Mimura MAM, Borra RC, Hirata CHW, De Oliveira N (2017) Immune response of patients with recurrent aphthous stomatitis challenged with a symbiotic. J Oral Pathol Med 46:821–828. https://doi.org/10.1111/jop.12621
Martins FS, Nardi RMD, Arantes RME, Rosa CA, Neves MJ, Nicoli JR (2005) Screening of yeast as probiotic based on capacities to colonize the gastrointestinal tract and to protect against enteropathogen challenge in mice. J Gen Appl Microbiol 51:83–92. https://doi.org/10.2323/jgam.51.83
Martins FS, Rodrigues ACP, Tiago FCP, Penna FJ, Rosa CA, Arantes RME, Nardi RMD, Neves MJ, Nicoli JR (2007) Saccharomyces cerevisiae strain 905 reduces the translocation of Salmonella enterica serotype Typhimurium and stimulates the immune system in gnotobiotic and conventional mice. J Med Microbiol 56:352–359. https://doi.org/10.1099/jmm.0.46525-0
Martins FS, Elian ABSDA, Vieira AT, Tiago FCP, Martins AKS, Silva FCP, Souza ÉLS, Sousa LP, Araújo HC, Pimenta PF, Bonjardim CA, Arantes RME, Teixeira MM, Nicoli JR (2011) Oral treatment with Saccharomyces cerevisiae strain UFMG 905 modulates immune responses and interferes with signal pathways involved in the activation of inflammation in a murine model of typhoid fever. Int J Med Microbiol 301:359–364. https://doi.org/10.1016/j.ijmm.2010.11.002
Tiago FCP, Martins FS, Souza ELS, Pimenta PFP, Araujo HRC, Castro IM, Branda RL, Nicoli JR (2012) Adhesion to the yeast cell surface as a mechanism for trapping pathogenic bacteria by Saccharomyces probiotics. J Med Microbiol 61:94–1207. https://doi.org/10.1099/jmm.0.042283-0
Generoso SV, Viana M, Santos R, Martins FS, Machado JA, Arantes RM, Nicoli JR, Correia MI, Cardoso VN (2010) Saccharomyces cerevisiae strain UFMG 905 protects against bacterial translocation, preserves gut barrier integrity and stimulates the immune system in a murine intestinal obstruction model. Arch Microbiol 192:477–484. https://doi.org/10.1007/s00203-010-0574-8
Bastos RW, Pedroso SH, Vieira AT, Moreira LM, França CS, Cartelle CT, Arantes RM, Generoso SV, Cardoso VN, Neves MJ, Nicoli JR, Martins FS (2016) Saccharomyces cerevisiae UFMG A-905 treatment reduces intestinal damage in a murine model of irinotecan-induced mucositis. Benef Microbes 7:549–557. https://doi.org/10.3920/BM2015.0190
Porto BAA, Monteiro CF, Souza EIS, Leocádio PCI, Alvarez-leite JJ, Generoso SV, Cardoso VN, Almeida-Leite CM, Santos DA, Santos JRA, Nicoli JR, Pessione E, Martins FS (2019) Treatment with selenium-enriched Saccharomyces cerevisiae UFMG A-905 partially ameliorates mucositis induced by 5-fluorouracil in mice. Cancer Chemother Pharmacol 84:117–126. https://doi.org/10.1007/s00280-019-03865-8
Tiago FC, Porto BA, Ribeiro NS, Moreira LM, Arantes RM, Vieira AT, Teixeira MM, Generoso SV, Nascimento VN, Martins FS, Nicoli JR (2015) Effect of Saccharomyces cerevisiae strain UFMG A-905 in experimental model of inflammatory bowel disease. Benef Microbes 6:807–815. https://doi.org/10.3920/BM2015.0018
Fonseca VMB, Milani TMS, Prado R, Bonato VLD, Ramos SG, Martins FS, Vianna EO, Borges MC (2017) Oral administration of Saccharomyces cerevisiae UFMG A-905 prevents allergic asthma in mice. Respirology 22:905–912. https://doi.org/10.1111/resp.12990
Miranda VC, Santos SS, Assis HC, Faria AMC, Quintanilha MF, Morão RP, Nicoli JR, Cara DC, Martins FS (2020) Effect of Saccharomyces cerevisiae UFMG A-905 in a murine model of food allergy. Benef Microbes 11:255–268. https://doi.org/10.3920/BM2019.0113
Coutinho JOPA, Peixoto TS, Menezes GCA, Carvalho CR, Ogaki MB, Gomes ECQ, Rosa CA, Rosa LH, Arantes RME, Nicoli JR, Tiago FCP, Martins FS (2021) In vitro and in vivo evaluation of the probiotic potential of Antarctic yeasts. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-021-09758-8
De Menezes GCA, Amorim SS, Gonçalves VN, Godinho VM, Simões JC, Rosa CA, Rosa LH (2019) Diversity, distribution, and ecology of fungi in the seasonal snow of Antarctica. Microorganisms 7:445. https://doi.org/10.3390/microorganisms7100445
Hof H (2019) Rhodotorula spp. in the gut – foe or friend? GMS Infectious Diseases 7:1–6. https://doi.org/10.3205/id000042
Navarrete P, Tovar-Ramírez D (2014) Use of yeasts as probiotics in fish aquaculture, in Sustainable aquaculture techniques, eds M. Hernandez-Vergara and C. Pérez-Rostro (Rijeka: InTech) 135–172.
Raggi P, Lopez P, Diaz A, Carrasco D, Silva A, Velez A, Opazo R, Magne F, Navarrete PA (2014) Debaryomyces hansenii and Rhodotorula mucilaginosa comprised the yeast core gut microbiota of wild and reared carnivorous salmonids, croaker and yellowtail. Environ Microbiol 16:2791–2803. https://doi.org/10.1111/1462-2920.12397
Sokół I, Gaweł A, Bobrek K (2018) The prevalence of yeast and characteristics of the isolates from the digestive tract of clinically healthy turkeys. Avian Dis 62:286–290. https://doi.org/10.1637/11780-121117
Kowalewska B, Zorena K, Szmigiero-kawko M, Wąż P, Myśliwiec M (2016) Higher diversity in fungal species discriminates children with type 1 diabetes mellitus from healthy control. Patient Prefer and Adher 10:591–599. https://doi.org/10.2147/PPA.S97852
Strati F, Di Paola M, Stefanini I, Albanese D, Rizzetto L, Lionetti P, Calabrò A, Jousson O, Donati C, Cavalieri D, De Filippo C (2016) Age and gender affect the composition of fungal population of the human gastrointestinal tract. Front microbiol 7:1227. https://doi.org/10.3389/fmicb.2016.01227
Hof H (2017) Pilze im Darm – das Mykobiom des Darms [Fungi in the gut – the gut mycobiome]. Gastroenterology 55:772–778. https://doi.org/10.1055/s-0043-112657
Chen XQ, Zhao W, Xie SW, Xie JJ, Zhang ZH, Tian LX, Liu YJ, Niu J (2019) Effects of dietary hydrolyzed yeast (Rhodotorula mucilaginosa) on growth performance, immune response, antioxidant capacity and histomorphology of juvenile Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 90:30–39. https://doi.org/10.1016/j.fsi.2019.03.068
Zhou C, Lin H, Xia D, Yang K, Yang Y, Yu W (2016) Effect of dietary marine red yeast Rhodotorula mucilaginosa on the growth performance, and also non-specific immune responses of juvenile golden Pompano trachinotus ovatus when challenged with Vibrio harveyi. Isr J Aquacult 68:1–9. https://doi.org/10.46989/001c.20829
Skrivan M, Marounek M, Englmaierova M, Skrivanova E (2016) Effect of increasing doses of marigold (Tagetes erecta) flower extract on eggs carotenoids content, colour and oxidative stability. J Anim Feed Sci 25:58–64. https://doi.org/10.22358/jafs/65588/2016
Sun JLM, Tang Z, Zhang X, Chen J, Sun Z (2019) Effects of Rhodotorula mucilaginosa fermentation product on the laying performance, egg quality, jejunal mucosal morphology and intestinal microbiota of hens. Appl Microbiol 128:54–64. https://doi.org/10.1111/jam.14467
Marova I, Carnecka M, Halienova A, Certik M, Dvorakova T, Haronikova A (2012) Use of several waste substrates for carotenoid-rich yeast biomass production. J Environ Manage 95:338–342. https://doi.org/10.1016/j.jenvman.2011.06.018
Karadas F, Grammenidis E, Surai PF, Acamovic T, Sparks C (2006) Effects of carotenoids from lucerne, marigold and tomato on egg yolk pigmentation and carotenoid composition. Br Poultry Sci 47:561–566. https://doi.org/10.1080/00071660600962976
Nicolle C, Cardinault N, Aprikian O, Busserolles J, Grolier P, Rock E, Demigne C, Mazur A (2003) Effect of carrot intake on cholesterol metabolism and on antioxidant status in cholesterol-fed rat. Eur J Nutr 42:254–261. https://doi.org/10.1007/s00394-003-0419-1
Kotrbacek V, Doucha J, Offenbartl T (2004) Use of Chlorella as a carrier of organic-bound iodine in the nutrition of sows. Czech J Anim Sci 49:28–32
Svoboda M, Kotrbacek V, Ficek R, Drabek J (2009) Effect of organic selenium from Se-enriched alga (Chlorella spp.) on selenium transfer from sows to their progeny. Acta Vet Brno 78:373–377. https://doi.org/10.1016/j.anifeedsci.2018.02.004
Hou W, Ma Z, Sun L, Han M, Lu J, Li Z, Abdalla O, Wei G (2013) Extracellular polymeric substances from copper-tolerance Sinorhizobium meliloti immobilize Cu2+. J Haz Mat 261:614–620. https://doi.org/10.1016/j.jhazmat.2013.06.043
Chapot-Chartier MP (2014) Interactions of the cell-wall glycopolymers of lactic acid bacteria with their bacteriophages. Front Microbiol 5:236. https://doi.org/10.3389/fmicb.2014.00236
Chiaro TR, Soto R, Stephens W, Kubinak JL, Petersen C, Gogokhia L, Bell R, Delgado JC, Cox J, Voth W, Brown J, Stillman DJ, O’connell RM, Tebo AE, Round JL, (2017) A member of the gut mycobiota modulates host purine metabolism exacerbating colitis in mice. Sci Transl Med 03:380. https://doi.org/10.1126/scitranslmed.aaf9044
Baradkar VP, Kumar S (2008) Meningitis caused by Rhodotorula mucilaginosa in human immunodeficiency virus seropositive patient. Ann Indian Acad Neurol 11:245–247. https://doi.org/10.4103/0972-2327.44561
Wirth F, Goldani LZ (2012) Epidemiology of Rhodotorula: an emerging pathogen. Interdiscip Perspect Infect Dis 1:7. https://doi.org/10.1155/2012/465717
Santos DDS, Calaça PRA, Porto ALF, de Souza PRE, de Freitas NSA, Cavalcanti VSMT (2020) What differentiates probiotic from pathogenic bacteria? The genetic mobility of Enterococcus faecium offers new molecular insights. OMICS: J Integrative Biol 12:706–713. https://doi.org/10.1089/omi.2020.0078
Souza ÉL, Elian SD, Paula LM, Garcia CC, Vieira AT, Teixeira MM, Arantes RM, Nicoli JR, Martins FS (2016) Escherichia coli strain Nissle 1917 ameliorates experimental colitis by modulating intestinal permeability, the inflammatory response and clinical signs in a faecal transplantation model. J Med Microbiol 65:201–210. https://doi.org/10.1099/jmm.0.000222
Vieira AT, Fagundes CT, Alessandri AL, Castor MGM, Guabiraba R, Borges VO, Teixeira MM (2009) Treatment with a novel chemokine-binding protein or eosinophil lineage-ablation protects mice from experimental colitis. Am J Clin Pathol 175:2382–2391. https://doi.org/10.2353/ajpath.2009.090093
Turner JR (2009) Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9:799–809. https://doi.org/10.1038/nri2653
Atıcı S, Soysal A, Karadeniz CK, Yılmaz Ş, Aksu B, Kıyan G, Bakır M (2017) Catheter-related Saccharomyces cerevisiae fungemia following Saccharomyces boulardii probiotic treatment: in a child in intensive care unit and review of the literature. Med Mycol Case Rep 15:33–35. https://doi.org/10.1016/j.mmcr.2017.02.002
Ponceleta A, Ruellea L, Konopnickia D, Miendje VY, Daubya DN (2021) Saccharomyces cerevisiae fungemia: risk factors, outcome and links with S. boulardii-containing probiotic administration. Infect Dis Now 51:293–295. https://doi.org/10.1016/j.idnow.2020.12.003
Albert E, Walker J, Thiesen A, Churchill T, Madsen K (2010) cis-Urocanic acid attenuates acute dextran sodium sulphate-induced intestinal inflammation. PLoS ONE 5:e13676. https://doi.org/10.1371/journal.pone.0013676
Cooper HS, Murthy SNS, Shah RS, Sedergran DJ (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Inv 69:238–249
Andrade ME, Santos RD, Soares AD, Costa KA, Fernandes SO, de Souza CM, Cassali GD, de Souza AL, Faria AM, Cardoso VN (2016) Pretreatment and treatment with l-arginine attenuate weight loss and bacterial translocation in dextran sulfate sodium colitis. J Parenter Enteral Nutr 40:1131–1139. https://doi.org/10.1177/0148607115581374
Souza DG, Cara DC, Cassali GD, Coutinho SF, Silveira MR, Andrade SP, Poole SP, Teixeira MM (2000) Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries following ischaemia of the superior mesenteric artery in the rat. Br J Pharmacol 131:1800–1808. https://doi.org/10.1038/sj.bjp.0703756
Soares PM, Mota JM, Gomes AS, Oliveira RB, Assreuy AM, Brito GA, Santos AA, Ribeiro RA, Souza MH (2008) Gastrointestinal dysmotility in 5-fluorouracil-induced intestinal mucositis outlasts inflammatory process resolution. Cancer Chemother Pharmacol 63:91–98. https://doi.org/10.1007/s00280-008-0715-9
Pedroso SH, Vieira AT, Bastos RW, Oliveira JS, Cartelle CT, Arantes RM, Soares PM, Generoso SV, Cardoso VN, Teixeira MM, Nicoli JR, Martins FS (2015) Evaluation of mucositis induced by irinotecan after microbial colonization in germ-free mice. Microbiol 161:1950–1960. https://doi.org/10.1099/mic.0.000149
Fata F, Ron IG, Kemeny N, O’Reilly E, Klimstra D, Kelsen DP (1999) 5-Fluorouracil-induced small bowel toxicity in patients with colorectal carcinoma. Cancer 86:1129–1134. https://doi.org/10.1002/(sici)1097-0142(19991001)86:7%3c1129::aid-cncr5%3e3.0.co;2-4
Tiago FCP, Martins FS, Rosa CA, Nardi RMD, Cara DC, Nicoli JR (2009) Physiological characterization of non-Saccharomyces yeasts from agro-industrial and environmental origins with possible probiotic function. World J Microbiol Biotechnol 25:657–666. https://doi.org/10.1007/s11274-008-9934-9
Eduardo FP, Bezinelli LM, Gobbi MF, Pereira AZ, Vogel C, Hamerschlak N, Corrêa L (2017) Impact of oral and gastrointestinal mucositis on body weight alterations during hematopoietic stem cell transplantation. Nutr Cancer 70:1–8. https://doi.org/10.1080/01635581.2018.1412476
Cheah KY, Howarth GS, Yazbeck R, Wright TH, Whitford EJ, Payne C, Butler RN, Bastian SEP (2009) Grape seed extract protects IEC-6 cells from chemotherapy-induced cytotoxicity and improves parameters of small intestinal mucositis in rats with experimentally-induced mucositis. Cancer Biol Ther 8:382–390. https://doi.org/10.4161/cbt.8.4.7453
Smith CL, Geier MS, Yazbeck R, Torres DM, Butler RN, Howarth GS (2008) Lactobacillus fermentum BR11 and fructo-oligosaccharide partially reduce jejunal inflammation in a model of intestinal mucositis in rats. Nutr Cancer 60:757–767. https://doi.org/10.1080/01635580802192841
De Barros PAV, Rabelo AME, de Vasconcelos GS, Mendes MSE, dos Reis DC, Lacerda LPC, Cardoso VN (2018) Conjugated linoleic acid prevents damage caused by intestinal mucositis induced by 5-fluorouracil in an experimental model. Biomed Pharmacother 103:1567–1576. https://doi.org/10.1016/j.biopha.2018.04.133
Kato S, Hamouda N, Kano Y, Oikawa Y, Tanaka Y, Matsumoto K, Shimakawa M (2017) Probiotic Bifidobacterium bifidum G9–1 attenuates 5-fluorouracil-induced intestinal mucositis in mice via suppression of dysbiosis related secondary inflammatory responses. Clin Exp Pharmacol Physiol 44:1017–1025. https://doi.org/10.1111/1440-1681.12792
Duncan M, Grant G (2003) Oral and intestinal mucositis – causes and possible treatments. Aliment Pharmacol Ther 18:853–874. https://doi.org/10.1046/j.1365-2036.2003.01784.x
Lee CS, Ryan EJ, Doherty GA (2014) Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: the role of inflammation. World J Gastroenterol 20:3751–3761. https://doi.org/10.3748/wjg.v20.i14.3751
Soares PMG, Mota JMSC, Souza EP, Justino PFC, Franco AX, Cunha FQ, Souza MHLP (2013) Inflammatory intestinal damage induced by 5-fluorouracil requires IL-4. Cytokine 61:46–49. https://doi.org/10.1016/j.cyto.2012.10.003
Li HL, Lu L, Wang XS, Qin LY, Wang P, Qiu SP, Wu XJ (2017) Alteration of gut microbiota and inflammatory cytokine/chemokine profiles in 5-fluorouracil induced intestinal mucositis. Front Cell Infect Microbiol 7:455. https://doi.org/10.3389/fcimb.2017.00455
Song MK, Park MY, Sung MK (2013) 5-Fluorouracil-induced changes of intestinal integrity biomarkers in BALB/c mice. J Cancer Prev 18:322–329. https://doi.org/10.15430/JCP.2013.18.4.322
Andrade ME, Araújo RS, de Barros PA, Soares AD, Abrantes FA, Generoso SV, Fernandes SO, Cardoso VN (2015) The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin Nutr 34:1080–1087. https://doi.org/10.1016/j.clnu.2015.01.012
Justino PFC, Melo LFM, Nogueira AF, Costa JVG, Silva LMN, Santos CM, Soares PMG (2014) Treatment with Saccharomyces boulardii reduces the inflammation and dysfunction of the gastrointestinal tract in 5-fluorouracil-induced intestinal mucositis in mice. Br J Nutr 111:1611–1621. https://doi.org/10.1017/S0007114513004248
Araújo CV, Lazzarotto CR, Aquino CC, Figueiredo IL, Costa TB, Alves LA, Ribeiro RA, Bertolini LR, Lima AAM, Brito GAC, Oria RB (2015) Alanyl-glutamine attenuates 5-fluorouracil-induced intestinal mucositis in apolipoprotein E-deficient mice. Braz J Med Biol Res 48:493–501. https://doi.org/10.1590/1414-431x20144360
Wang C, Liu Y, Sun G, Li X, Liu Z (2018) Growth, immune response, antioxidant capability, and disease resistance of juvenile Atlantic salmon (Salmo salar L.) fed Bacillus velezensis V4 and Rhodotorula mucilaginosa. Aquaculture 500:65–74. https://doi.org/10.1016/j.aquaculture.2018.09.052
Garza-Cervantes JA, Escárcega-González CE, Castro EDB, Mendiola-Garza G, Marichal-Cancino BA, López-Vázquez MA, Morones-Ramirez JR (2019) Antimicrobial and antibiofilm activity of biopolymer-Ni, Zn nanoparticle biocomposites synthesized using R. mucilaginosa UANL-001L exopolysaccharide as a capping agent. Int J Nanomedicine 4:2557–2571. https://doi.org/10.2147/IJN.S196470
Hamidi M, Gholipour AR, Delattre C, Sesdighi F, Seveiri RM, Pasdaran A, Kheirandish S, Pierre G, Kozani PS, Kozani PS, Karimitabar F (2020) Production, characterization and biological activities of exopolysaccharides from a new cold-adapted yeast: Rhodotorula mucilaginosa sp. GUMS16. Int J Biol Macromol 151:268–277. https://doi.org/10.1016/j.ijbiomac.2020.02.206
Funding
This work was supported by grants from the Minas Gerais State Research Support Foundation (FAPEMIG, APQ-00593–14) and the Coordination for the Improvement of Higher Education Personnel (CAPES 88887.136384/2017–00 and 88887.314457/2019–00). Financial support was also received from CNPq PROANTAR 442258/2018–6, INCT Criosfera II, and FNDCT. JOPAC was the recipient of a doctorate’s fellowship from CAPES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Declarations
All animal procedures were carried out according to the standards of the Brazilian Society of Laboratory Animal Science/Brazilian College for Animal Experimentation (available at http://www.mctic.gov.br/concea). This work was approved by the Ethics Committee in Animal Experimentation of the Federal University of Minas Gerais (CEUA/UFMG, protocol # 186/2012).
Disclaimer
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Coutinho, J.O.P.A., Quintanilha, M.F., Campos, M.R.A. et al. Antarctic Strain of Rhodotorula mucilaginosa UFMGCB 18,377 Attenuates Mucositis Induced by 5-Fluorouracil in Mice. Probiotics & Antimicro. Prot. 14, 486–500 (2022). https://doi.org/10.1007/s12602-021-09817-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09817-0