Abstract
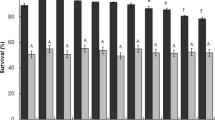
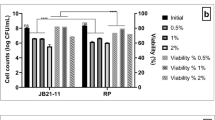
Weissella paramesenteroides has gained a considerable attention as bacteriocin and exopolysaccharide producers. However, potential of W. paramesenteroides to utilize different prebiotics is unexplored area of research. Fruits being vectors of various probiotics, five W. paramesenteroides strains, namely, FX1, FX2, FX5, FX9, and FX12, were isolated from different fruits. They were screened and selected based on their ability to survive at pH 2.5 and in 1.0% sodium taurocholate, high cell surface hydrophobicity, mucin adhesion, bile-induced biofilm formation, antimicrobial activity (AMA) against selected enteropathogens, and prebiotic utilization ability, implicating the functional properties of these strains. In vitro safety evaluation showed that strains were susceptible to antibiotics except vancomycin and did not harbor any virulent traits such as biogenic amine production, hemolysis, and DNase production. Based on their functionality, two strains FX5 and FX9 were selected for prebiotic utilization studies by thin layer chromatography (TLC) and short-chain fatty acids (SCFAs) production by high performance liquid chromatography. TLC profile evinced the ability of these two strains to utilize low molecular weight galactooligosaccharides (GOS) and fructooligosaccharides (FOS), as only the upper low molecular weight fractions were disappeared from cell-free-supernatants (CFS). Enhanced β-galactosidase activity correlated with galactose accumulation in residual CFS of GOS displayed GOS utilization ability. Both the strains exhibited AMA against E. coli and Staph. aureus and high SCFAs production in the presence of prebiotic, suggesting their synbiotic potential. Thus, W. paramesenteroides strains FX5 and FX9 exhibit potential probiotic properties with prebiotic utilization and can be taken forward to evaluate synergistic synbiotic potential in detail.




Similar content being viewed by others
References
Ljungh A, Wadström T (2006) Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol 7:73–89
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. https://doi.org/10.1038/nrgastro.2014.66
Lievin-Le Moal V, Servin AL (2014) Anti-infective activities of lactobacillus strains in the human intestinal microbiota: from probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin Microbiol Rev 27:167–199. https://doi.org/10.1128/CMR.00080-13
Lee CS, Kim SH (2019) Anti-inflammatory and anti-osteoporotic potential of Lactobacillus plantarum A41 and L. fermentum SRK414 as probiotics. Probiotics Antimicrob Proteins 2:1–12. https://doi.org/10.1007/s12602-019-09577-y
Shukla G, Kamboj S, Sharma B (2019) Comparative analysis of antigiardial potential of heat inactivated and probiotic protein of probiotic Lactobacillus rhamnosus GG in murine giardiasis. Probiotics Antimicrob Proteins 4:1–9. https://doi.org/10.1007/s12602-018-9506-8
Fusco V, Quero GM, Cho GS, Kabisch J, Meske D, Neve H, Bockelmann W, Franz CMAP (2015) The genus Weissella: taxonomy, ecology and biotechnological potential. Front Microbiol 6:155. https://doi.org/10.3389/fmicb.2015.00155
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G (2017) Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14:491–502. https://doi.org/10.1038/nrgastro.2017.75
Swennen K, Courtin CM, Delcour JA (2006) Non-digestible oligosaccharides with prebiotic properties. Crit Rev Food Sci Nutr 46:459–471. https://doi.org/10.1080/10408390500215
Goh YJ, Klaenhammer TR (2015) Genetic mechanisms of prebiotic oligosaccharide metabolism in probiotic microbes. Annu Rev Food Sci Technol 6:137–156. https://doi.org/10.1146/annurev-food-022814-015706
Veereman-Wauters G, Staelens S, Van de Broek H, Plaskie K, Wesling F, Roger L, McCartney A, Assam P (2011) Physiological and bifidogenic effects of prebiotic supplements in infant formulae. J Pediatr Gastroenterol Nutr 52:763–771. https://doi.org/10.1097/mpg.0b013e3182139f39
Kolida S, Gibson GR (2011) Synbiotics in health and disease. Annu Rev Food Sci Technol 2:373–393. https://doi.org/10.1146/annurev-food-022510-133739
Fessard A, Remize F (2017) Why are Weissella spp. not used as commercial starter cultures for food fermentation. Fermentation 3:38. https://doi.org/10.3390/fermentation3030038
Pithva S, Shekh S, Dave J, Vyas BRM (2014) Probiotic attributes of autochthonous Lactobacillus rhamnosus strains of human origin. Appl Biochem Biotechnol 173:259–277. https://doi.org/10.1007/s12010-014-0839-9
Singh S, Bhatia R, Singh A, Singh P, Kaur R, Khare P, Purama RK, Boparai RK, Rishi P, Ambalam P, Bhadada SK, Bishnoi M, Kaur J, Kondepudi KK (2018) Probiotic attributes and prevention of LPS-induced pro-inflammatory stress in RAW264.7 macrophages and human intestinal epithelial cell line (Caco-2) by newly isolated Weissella cibaria strains. Food Funct 9:1254–1264. https://doi.org/10.1039/c7fo00469a
Kondepudi KK, Ambalam P, Nilsson I, Wadström T, Ljungh A (2012) Prebiotic-non-digestible oligosaccharides preference of probiotic bifidobacteria and antimicrobial activity against Clostridium difficile. Anaerobe 18:489–497. https://doi.org/10.1016/j.anaerobe.2012.08.005
Lindahl M, Faris A, Wadstrom T, Hjerten S (1981) A new test based on ‘salting out’ to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta 77:471–476. https://doi.org/10.1016/0304-4165(81)90261-0
Campana R, van Hemert S, Baffone W (2017) Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog 9:12. https://doi.org/10.1186/s13099-017-0162-4
Ambalam P, Kondepudi KK, Nilsson I, Wadstrom T, Ljungh Å (2012) Bile stimulates cell surface hydrophobicity, Congo red binding and biofilm formation of Lactobacillus strains. FEMS Microbiol Lett 333:10–19. https://doi.org/10.1111/j.1574-6968.2012.02590.x
Valeriano VD, Parungao-Balolong MM, Kang DK (2012) In vitro evaluation of the mucin-adhesion ability and probiotic potential of Lactobacillus mucosae LM1. J Appl Microbiol 117:485–497. https://doi.org/10.1111/jam.12539
Jeffries CD, Holtman DF, Guse DG (1957) Rapid method for determining the activity of microorganisms on nucleic acids. J Bacteriol 73:590–591
Thongaram T, Hoeflinger JL, Chow J, Miller MJ (2017) Human milk oligosaccharide consumption by probiotic lactobacilli and bifidobacteria. J Agric Food Chem 100:7825–7833. https://doi.org/10.3168/jds.2017-12753
Ambalam P, Kondepudi KK, Balusupati P, Nilsson I, Wadstrom T, Ljungh Å (2015) Prebiotic preferences of human lactobacilli strains in co-culture with bifidobacteria and antimicrobial activity against Clostridium difficile. J Appl Microbiol 119:1672–1682. https://doi.org/10.1111/jam.12953
Delzenne NM (2003) Oligosaccharides: state of the art. Proc Nutr Soc 62:177–182. https://doi.org/10.1079/PNS2002225
Collins MD, Samelis J, Metaxopoulos J, Wallbanks S (1993) Taxonomic studies on some leuconostoc-like organisms from fermented sausages: description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J Appl Microbiol 75:595–603. https://doi.org/10.1111/j.1365-2672.1993.tb01600.x
Pal A, Ramana KV (2009) Isolation and preliminary characterization of a non bacteriocin antimicrobial compound from Weissella paramesenteroides DFR-8 isolated from cucumber (Cucumis sativus). Process Biochem 44:499–503. https://doi.org/10.1016/j.procbio.2009.01.006
Ku HJ, Kim YT, Lee JH (2017) Genomic insights of Weissella jogaejeotgali FOL01T reveals its food fermentation ability and human gut adaptive potential for probiotic applications in food industries. J Microbiol Biotechnol 27:943–946. https://doi.org/10.4014/jmb.1702.02054
Wang L, Si W, Xue H, Zhao X (2017) A fibronectin-binding protein (FbpA) of Weissella cibaria inhibits colonization and infection of Staphylococcus aureus in mammary glands. Cell Microbiol 19:e12731. https://doi.org/10.1111/cmi.12731
Xiong L, Ni X, Niu L, Zhou Y, Wang Q, Khalique A, Liu Q, Zeng Y, Shu G, Pan K, Jing B (2019) Isolation and preliminary screening of a Weissella confusa strain from giant panda (Ailuropoda melanoleuca). Probiotics Antimicrob Proteins 11:535–544. https://doi.org/10.1007/s12602-018-9402-2
Lambert JM, Bongers RS, de Vos WM, Kleerebezem M (2008) Functional analysis of four bile salt hydrolase and penicillin acylase family members in Lactobacillus plantarum WCFS1. Appl Environ Microbiol 74:4719–4726. https://doi.org/10.1128/aem.00137-08
Ruiz L, Margolles A, Sánchez B (2013) Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol 4:396. https://doi.org/10.3389/fmicb.2013.00396
Bibiloni R, Perez PF, Garrote GL, Disalvo EA, De Antoni GL (2001) Surface characterization and adhesive properties of bifidobacteria. Methods Enzymol 336:411–427. https://doi.org/10.1016/s0076-6879(01)36605-3
Canzi E, Guglielmetti S, Mora D, Tamagnini I, Parini C (2005) Conditions affecting cell surface properties of human intestinal bifidobacteria. Antonie Van Leeuwenhoek 88:207–219. https://doi.org/10.1007/s10482-005-6501-3
Ramiah K, van Reenen CA, Dicks LM (2009) Expression of the mucus adhesion gene mub, surface layer protein slp and adhesion-like factor EF-TU of Lactobacillus acidophilus ATCC 4356 under digestive stress conditions, as monitored with real-time PCR. Probiotics Antimicrob Proteins 1:91–95. https://doi.org/10.1007/s12602-009-9009-8
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890. https://doi.org/10.3201/eid0809.020063
Ambalam P, Kondepudi KK, Nilsson I, Wadström T, Ljungh Å (2014) Bile enhances cell surface hydrophobicity and biofilm formation of bifidobacteria. Appl Biochem Biotechnol 172:1970–1981. https://doi.org/10.1007/s12010-013-0596-1
Joint FAO (2002) Guidelines for the evaluation of probiotics in food, London, Ontario, Canada. April 30 and May 1, 2002
Mathur S, Singh R (2005) Antibiotic resistance in food lactic acid bacteria-a review. Int J Food Microbiol 105:281–295. https://doi.org/10.1016/j.ijfoodmicro.2005.03.008
Jeong DW, Lee JH (2015) Antibiotic resistance, hemolysis and biogenic amine production assessments of Leuconostoc and Weissella isolates for kimchi starter development. LWT Food Sci Technol 64:1078–1084. https://doi.org/10.1016/j.lwt.2015.07.031
Garai G, Dueñas MT, Irastorza A, Moreno-Arribas MV (2007) Biogenic amine production by lactic acid bacteria isolated from cider. J Appl Microbiol 45:473–478. https://doi.org/10.1111/j.1472-765X.2007.02207.x
Lee KW, Park JY, Jeong HR, Heo HJ, Han NS, Kim JH (2012) Probiotic properties of Weissella strains isolated from human faeces. Anaerobe 18:96–102. https://doi.org/10.1016/j.anaerobe.2011.12.015
Andersen JM, Barrangou R, Abou Hachem M, Lahtinen SJ, Goh YJ, Svensson B, Klaenhammer TR (2013) Transcriptional analysis of oligosaccharide utilization by Bifidobacterium lactis Bl-04. BMC Genomics 14:312. https://doi.org/10.1186/1471-2164-14-312
Perrin S, Fougnies C, Grill JP, Jacobs H, Schneider F (2002) Fermentation of chicory fructooligosaccharides in mixtures of different degrees of polymerization by three strains of bifidobacteria. Can J Microbiol 48:759–763. https://doi.org/10.1139/w02-065
Watson D, O'Connell MM, Schoterman MH, van Neerven RJ, Nauta A, Van Sinderen D (2012) Selective carbohydrate utilization by lactobacilli and bifidobacteria. J Appl Microbiol 114:1132–1146. https://doi.org/10.1111/jam.12105
Macfarlane GT, Macfarlane S (2011) Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol 45:S120–S127. https://doi.org/10.1097/mcg.0b013e31822fecfe
Funding
This work was funded by the University Grant Commission (UGC), Government of India, under Major Research Project Scheme (MRP-Major-MICR-2013-35872). Ms. Kinjal Pabari is thankful to UGC for providing research fellowship. Authors would like to thank Center of Excellence (CoE) in Drug Discovery, Saurashtra University, Gujarat, for providing facility for HPLC analysis. Authors are grateful to Friesland Campina, the Netherlands for providing Vivinal GOS and Sweet Town Biotech, Taiwan for providing XOS.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Pabari, K., Pithva, S., Kothari, C. et al. Evaluation of Probiotic Properties and Prebiotic Utilization Potential of Weissella paramesenteroides Isolated From Fruits. Probiotics & Antimicro. Prot. 12, 1126–1138 (2020). https://doi.org/10.1007/s12602-019-09630-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-019-09630-w