Abstract
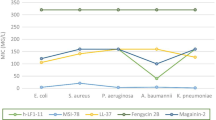
While nisin (lantibiotic), lacticin 3147 (lantibiotic) and vancomycin (glycopeptides) are among the best studied lipid II-binding antimicrobials, their relative activities have never been compared. Nisin and lacticin 3147 have been employed/investigated primarily as food preservatives, although they do have potential in terms of veterinary and clinical applications. Vancomycin is used exclusively in clinical therapy. We reveal a higher potency for lacticin 3147 (MIC 0.95–3.8 μg/ml) and vancomycin (MIC 0.78–1.56 μg/ml) relative to that of nisin (MIC 6.28–25.14 μg/ml) against the food-borne pathogen Listeria monocytogenes. A comparison of the activity of the three antimicrobials against nisin resistance mutants of L. monocytogenes also reveals that their susceptibility to vancomycin and lacticin 3147 changed only slightly or not at all. A further assessment of relative activity against a selection of Bacillus cereus, Enterococcus and Staphylococcus aureus targets revealed that vancomycin MICs consistently ranged between 0.78 and 1.56 μg/ml against all but one strain. Lacticin 3147 was found to be more effective than nisin against B. cereus (lacticin 3147 MIC 1.9–3.8 μg/ml; nisin MIC 4.1–16.7 μg/ml) and E. faecium and E. faecalis targets (lacticin 3147 MIC from 1.9 to 3.8 μg/ml; nisin MIC ≥8.3 μg/ml). The greater effectiveness of lacticin 3147 is even more impressive when expressed as molar values. However, in agreement with the previous reports, nisin was the more effective of the two lantibiotics against S. aureus strains. This study highlights that in many instances the antimicrobial activity of these leading lantibiotics are comparable with that of vancomycin and emphasizes their particular value with respect to use in situations including foods and veterinary medicine, where the use of vancomycin is not permitted.
Similar content being viewed by others
References
Allerberger F, Wagner M (2010) Listeriosis: a resurgent foodborne infection. Clin Microbiol Infec 16(1):16–23
Arnesen LA, Granum PE, Buisson C, Bohlin J, Nielsen-LeRoux C (2011) Using an insect model to assess correlation between temperature and virulence in Bacillus weihenstephanensis and Bacillus cereus. FEMS Microbiol Lett 317(2):196–202
Barna JC, Williams DH (1984) The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu Rev Microbiol 38:339–357
Begley M, Hill C, Ross RP (2006) Tolerance of Listeria monocytogenes to cell envelope-acting antimicrobial agents is dependent on SigB. Appl Environ Microb 72(3):2231–2234
Bierbaum G, Sahl HG (2009) Lantibiotics: mode of action, biosynthesis and bioengineering. Curr Pharm Biotechnol 10(1):2–18
Boakes S, Cortes J, Appleyard AN, Rudd BA, Dawson MJ (2009) Organization of the genes encoding the biosynthesis of actagardine and engineering of a variant generation system. Mol Microbiol 72(5):1126–1136
Bottone EJ (2010) Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev 23(2):382–391
Breukink E, de Kruijff B (2006) Lipid II as a target for antibiotics. Nat Rev Drug Discov 5(4):321–332
Broadbent JR, Chou YC, Gillies K, Kondo JK (1989) Nisin inhibits several gram-positive, mastitis-causing pathogens. J Dairy Sci 72(12):3342–3345
Brotz H, Josten M, Wiedemann I, Schneider U, Gotz F, Bierbaum G, Sahl HG (1998) Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol Microbiol 30(2):317–327
Castiglione F, Cavaletti L, Losi D, Lazzarini A, Carrano L, Feroggio M, Ciciliato I, Corti E, Candiani G, Marinelli F, Selva E (2007) A novel lantibiotic acting on bacterial cell wall synthesis produced by the uncommon actinomycete Planomonospora sp. Biochemistry-US 46(20):5884–5895
Castiglione F, Lazzarini A, Carrano L, Corti E, Ciciliato I, Gastaldo L, Candiani P, Losi D, Marinelli F, Selva E, Parenti F (2008) Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multiresistant pathogens. Chem Biol 15(1):22–31
Collins B, Curtis N, Cotter PD, Hill C, Ross RP (2010) The ABC transporter AnrAB contributes to the innate resistance of Listeria monocytogenes to nisin, bacitracin, and various beta-lactam antibiotics. Antimicrob Agents Ch 54(10):4416–4423
Collins B, Joyce S, Hill C, Cotter PD, Ross RP (2010) TelA contributes to the innate resistance of Listeria monocytogenes to nisin and other cell wall-acting antibiotics. Antimicrob Agents Ch 54(11):4658–4663
Cotter PD, Emerson N, Gahan CGM, Hill C (1999) Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J Bacteriol 181(21):6840–6843
Cotter PD, Guinane CM, Hill C (2002) The LisRK signal transduction system determines the sensitivity of Listeria monocytogenes to nisin and cephalosporins. Antimicrob Ag Ch 46(9):2784–2790
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3(10):777–788
Cotter PD, Hill C, Ross RP (2005) Bacterial lantibiotics: strategies to improve therapeutic potential. Curr Prot Pep Sc 6(1):61–75
Cotter PD, Deegan LH, Lawton EM, Draper LA, O’Connor PM, Hill C, Ross RP (2006) Complete alanine scanning of the two-component lantibiotic lacticin 3147: generating a blueprint for rational drug design. Mol Microbiol 62(3):735–747
Cotter PD, Draper LA, Lawton EM, McAuliffe O, Hill C, Ross RP (2006) Overproduction of wild-type and bioengineered derivatives of the lantibiotic lacticin 3147. Appl Environ Microb 72(6):4492–4496
Courvalin P (2006) Vancomycin resistance in gram-positive cocci. Clin Infect Dis 42(Suppl 1):S25–S34
Crispie F, Twomey D, Flynn J, Hill C, Ross P, Meaney W (2005) The lantibiotic lacticin 3147 produced in a milk-based medium improves the efficacy of a bismuth-based teat seal in cattle deliberately infected with Staphylococcus aureus. J Dairy Res 72(2):159–167
De Buyser M-L, Dufour B, Maire M, Lafarge V (2001) Implication of milk and milk products in food-borne diseases in France and in different industrialised countries. Int J Food Microbiol 67(1–2):1–17
Deegan LH, Suda S, Lawton EM, Draper LA, Hugenholtz F, Peschel A, Hill C, Cotter PD, Ross RP (2006) Manipulation of charged residues within the two-peptide lantibiotic lacticin 3147. Microb Biotechnol 3(2):222–234
Ducey TF, Page B, Usgaard T, Borucki MK, Pupedis K, Ward TJ (2007) A single-nucleotide-polymorphism-based multilocus genotyping assay for subtyping lineage I isolates of Listeria monocytogenes. Appl Environ Microb 73(1):133–147
Ernst CM, Peschel A (2011) Broad-spectrum antimicrobial peptide resistance by MprF-mediated aminoacylation and flipping of phospholipids. Mol Microbiol 80(2):290–299
Field D, Connor PM, Cotter PD, Hill C, Ross RP (2008) The generation of nisin variants with enhanced activity against specific gram-positive pathogens. Mol Microbiol 69(1):218–230
Field D, Quigley L, O’Connor PM, Rea MC, Daly K, Cotter PD, Hill C, Ross RP (2010) Studies with bioengineered Nisin peptides highlight the broad-spectrum potency of Nisin V. Microb Biotechnol 3(4):473–486
Galvez A, Lopez RL, Abriouel H, Valdivia E, Omar NB (2008) Application of bacteriocins in the control of foodborne pathogenic and spoilage bacteria. Crit Rev Biotechnol 28(2):125–152
Galvin M, Hill C, Ross RP (1999) Lacticin 3147 displays activity in buffer against gram-positive bacterial pathogens which appear insensitive in standard plate assays. Lett Appl Microbiol 28(5):355–358
Giraffa G (2002) Enterococci from foods. FEMS Microbiol Rev 26(2):163–171
Guinane CM, Cotter PD, Hill C, Ross RP (2006) Spontaneous resistance in Lactococcus lactis IL1403 to the lantibiotic lacticin 3147. FEMS Microbiol Lett 260(1):77–83
Hsu ST, Breukink E, Tischenko E, Lutters MA, de Kruijff B, Kaptein R, Bonvin AM, van Nuland NA (2004) The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat Struct Mol Biol 11(10):963–967
King A, Phillips I (2001) The in vitro activity of daptomycin against 514 Gram-positive aerobic clinical isolates. J Antimicrob Ch 48(2):219–223
Klein G (2003) Taxonomy, ecology and antibiotic resistance of enterococci from food and the gastro-intestinal tract. Int J Food Microbiol 88(2–3):123–131
Klostermann K, Crispie F, Flynn J, Meaney WJ, Ross RP, Hill C (2010) Efficacy of a teat dip containing the bacteriocin lacticin 3147 to eliminate gram-positive pathogens associated with bovine mastitis. J Dairy Res 77(2):231–238
Kramer NE, Smid EJ, Kok J, de Kruijff B, Kuipers OP, Breukink E (2004) Resistance of gram-positive bacteria to nisin is not determined by Lipid II levels. FEMS Microbiol Lett 239(1):157–161. doi:10.1016/j.femsle.2004.08.033
Lindback T, Hardy SP, Dietrich R, Sodring M, Didier A, Moravek M, Fagerlund A, Bock S, Nielsen C, Casteel M, Granum PE, Martlbauer E (2010) Cytotoxicity of the Bacillus cereus Nhe enterotoxin requires specific binding order of its three exoprotein components. Infect Immun 78(9):3813–3821
Mandin P, Fsihi H, Dussurget O, Vergassola M, Milohanic E, Toledo-Arana A, Lasa I, Johansson J, Cossart P (2005) VirR, a response regulator critical for Listeria monocytogenes virulence. Mol Microbiol 57(5):1367–1380
Martin-Belloso O, Raybaudi-Massilia RM, Mosqueda-Melgar J, Sobrino-Lopez A, Soliva-Fortuny R (2007) Shelf-life extension of fresh-cut “Fuji” apples at different ripeness stages using natural substances. Postharvest Biol and Tec 45(2):265–275
McAuliffe O, Ross RP, Hill C (2001) Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol Rev 25(3):285–308
McGowan LL, Jackson CR, Barrett JB, Hiott LM, Fedorka-Cray PJ (2006) Prevalence and antimicrobial resistance of enterococci isolated from retail fruits, vegetables, and meats. J Food Prot 69(12):2976–2982
Mondino SSB, Castro ACD, Mondino PJJ, Carvalho MDS, Silva KMF, Teixeira LM (2003) Phenotypic and genotypic characterization of clinical and intestinal enterococci isolated from inpatients and outpatients in two Brazilian hospitals. Microb Drug Resist 9(2):167–174
Mootz HD, Kessler N, Linne U, Eppelmann K, Schwarzer D, Marahiel MA (2002) Decreasing the ring size of a cyclic nonribosomal peptide antibiotic by in-frame module deletion in the biosynthetic genes. J Am Chem Soc 124(37):10980–10981
Morgan SM, Galvin M, Ross RP, Hill C (2001) Evaluation of a spray-dried lacticin 3147 powder for the control of Listeria monocytogenes and Bacillus cereus in a range of food systems. Lett Appl Microbiol 33(5):387–391
Morgan SM, O’Connor PM, Cotter PD, Ross RP, Hill C (2005) Sequential actions of the two component peptides of the lantibiotic lacticin 3147 explain its antimicrobial activity at nanomolar concentrations. Antimicrob Agents Ch 49(7):2606–2611
Mota-Meira M, Lacroix C, LaPointe G, Lavoie MC (1997) Purification and structure of mutacin B-Ny266: a new lantibiotic produced by Streptococcus mutans. FEBS Lett 410(2–3):275–279
Mota-Meira M, LaPointe G, Lacroix C, Lavoie MC (2000) MICs of mutacin B-Ny266, nisin A, vancomycin, and oxacillin against bacterial pathogens. Antimicrob Agents Ch 44(1):24–29
Mota-Meira M, Morency H, Lavoie MC (2005) In vivo activity of mutacin B-Ny266. J Antimicrob Ch 56(5):869–871
Niu WW, Neu HC (1991) Activity of Mersacidin, a novel peptide, compared with that of vancomycin, Teicoplanin, and Daptomycin. Antimicrob Agents Ch 35(5):998–1000
O’Mahony J, Carroll J, Draper LA, O’Connor PM, Coffey A, Hill C, Ross RP, Cotter PD (2010) Comparison of the activities of the lantibiotics nisin and lacticin 3147 against clinically significant mycobacteria. Int J Antimicrob Ag 36(2):132–136
Piper C, Cotter PD, Draper LA, Ross RP, Hill C (2009) A comparison of the activities of lacticin 3147 and nisin against drug-resistant Staphylococcus aureus and Enterococcus species. J Antimicrob Chemoth 63(3):546–551
Piper C, Cotter PD, Ross RP, Hill C (2009) Discovery of medically significant lantibiotics. Curr Drug Discov Technol 6(1):1–18
Rivera AM, Boucher HW (2011) Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc 86(12):1230–1242. doi:10.4065/mcp2011.0514
Ross RP, Morgan S, Hill C (2002) Preservation and fermentation: past, present and future. Int J Food Microbiol 79(1):3–16
Ross RP, Rea MC, Clayton E, O’Connor PM, Shanahan F, Kiely B, Hill C (2007) Antimicrobial activity of lacticin 3147 against clinical Clostridium difficile strains. J Med Microbiol 56(7):940–946
Ruzin A, Severin A, Moghazeh SL, Etienne J, Bradford PA, Projan SJ, Shlaes DM (2003) Inactivation of mprF affects vancomycin susceptibility in Staphylococcus aureus. BBA-Gen Subjects 1621(2):117–121
Scannell AG, Ross RP, Hill C, Arendt EK (2000) An effective lacticin biopreservative in fresh pork sausage. J Food Prot 63(3):370–375
Schlech WF 3rd (2000) Foodborne listeriosis. Clin Infect Dis 31(3):770–775
Smith TL, Pearson ML, Wilcox KR, Cruz C, Lancaster MV, Robinson-Dunn B, Tenover FC, Zervos MJ, Band JD, White E, Jarvis WR, Staphylococ GI (1999) Emergence of vancomycin resistance in Staphylococcus aureus. New Engl J Med 340(7):493–501
Stack HM, Sleator RD, Bowers M, Hill C, Gahan CG (2005) Role for HtrA in stress induction and virulence potential in Listeria monocytogenes. Appl Environ Microb 71(8):4241–4247
Suda S, Cotter PD, Hill C, Ross RP (2011) Lacticin 3147: biosynthesis, molecular analysis, immunity, bioengineering and applications. Curr Prot Pep Sc (in print)
Sussmuth RD, Wohlleben W (2004) The biosynthesis of glycopeptide antibiotics–a model for complex, non-ribosomally synthesized, peptidic secondary metabolites. Appl Microbiol Biotechnol 63(4):344–350
Thedieck K, Hain T, Mohamed W, Tindall BJ, Nimtz M, Chakraborty T, Wehland J, Jansch L (2006) The MprF protein is required for lysinylation of phospholipids in listerial membranes and confers resistance to cationic antimicrobial peptides (CAMPs) on Listeria monocytogenes. Mol Microbiol 62(5):1325–1339
Upton M, Al-Mahrous MM (2011) Discovery and development of lantibiotics; antimicrobial agents that have significant potential for medical application. Expert Opin Drug Disc 6(2):155–170
Viguier C, Arora S, Gilmartin N, Welbeck K, O’Kennedy R (2009) Mastitis detection: current trends and future perspectives. Trends Biotechnol 27(8):486–493
Walsh FM, Amyes SG (2004) Microbiology and drug resistance mechanisms of fully resistant pathogens. Curr Opin Microbiol 7(5):439–444. doi:10.1016/j.mib.2004.08.007
Watanabe S, Kobayashi N, Quinones D, Hayakawa S, Nagashima S, Uehara N, Watanabe N (2009) Genetic diversity of the low-level vancomycin resistance gene vanC-2/vanC-3 and identification of a novel vanC subtype (vanC-4) in Enterococcus casseliflavus. Microb Drug Resist 15(1):1–9
Wiedemann I, Bottiger T, Bonelli RR, Wiese A, Hagge SO, Gutsmann T, Seydel U, Deegan L, Hill C, Ross P, Sahl HG (2006) The mode of action of the lantibiotic lacticin 3147–a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol Microbiol 61(2):285–296
Acknowledgments
This study was supported by funding (Project SOP HRD—SIMBAD 6853, 1.5/S/15—01.10.2008) to Catalin Iancu to facilitate an internship at the Microbiology Department, University College Cork, Ireland and by the Irish Government under the National Development Plan through a Science Foundation Ireland Investigator award to C.H., R.P.R and P.D.C. (06/IN.1/B98). The authors thank Mary Rea for providing indicator strains.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Iancu, C., Grainger, A., Field, D. et al. Comparison of the Potency of the Lipid II Targeting Antimicrobials Nisin, Lacticin 3147 and Vancomycin Against Gram-Positive Bacteria. Probiotics & Antimicro. Prot. 4, 108–115 (2012). https://doi.org/10.1007/s12602-012-9095-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-012-9095-x