Abstract
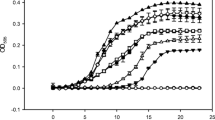
Subtilosin A is a 35-amino acid long cyclical peptide produced by Bacillus amyloliquefaciens that has potent antimicrobial activity against a variety of human pathogens, including the bacterial vaginosis-related Gardnerella vaginalis. The specific mode of action of subtilosin against G. vaginalis was elucidated by studying its effects on the proton motive force’s (PMF) components: transmembrane electric potential (∆Ψ), transmembrane pH gradient (ΔpH), and intracellular ATP levels. The addition of subtilosin to G. vaginalis cells caused an immediate and total depletion of the ΔpH, but had no effect on the ∆Ψ. Subtilosin also triggered an instant but partial efflux of intracellular ATP that was twofold higher than that of the positive control bacteriocin, nisin. Taken together, these data suggest that subtilosin inhibits G. vaginalis growth by creating transient pores in the cells’ cytoplasmic membrane, leading to an efflux of intracellular ions and ATP and eventually cell death.



Similar content being viewed by others
References
Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK (1983) Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74:14–22
Babasaki K, Takao T, Shimonishi Y, Kurahashi K (1985) Subtilosin A, a new antibiotic peptide produced by Bacillus subtilis 168: isolation, structural analysis, and biogenesis. J Biochem 98:585–603
Bannatyne RM, Smith AM (1998) Recurrent bacterial vaginosis and metronidazole resistance in Gardnerella vaginalis. Sex Transm Infect 74:455–456
Bauer R, Chikindas ML, Dicks LMT (2005) Purification, partial amino acid sequence and mode of action of pediocin PD-1, a bacteriocin produced by Pediococcus damnosus NCFB 1832. Int J Food Microbiol 101:17–27
Chikindas ML, García-Garcerá MJ, Driessen AJ, Ledeboer AM, Nissen-Meyer J, Nes IF, Abee T, Konings WN, Venema G (1993) Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl Environ Microbiol 59:3577–3584
Christensen DP, Hutkins RW (1992) Collapse of the proton motive force in Listeria monocytogenes caused by a bacteriocin produced by Pediococcus acidilactici. Appl Environ Microbiol 58:3312–3315
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 10:777–788
Eckert LO, Moore DE, Patton DL, Agnew KJ, Eschenbach DA (2003) Relationship of vaginal bacteria and inflammation with conception and early pregnancy loss following in vitro fertilization. Infect Dis Obstet Gynecol 11:11–17
Falagas ME, Betsi GI, Athanasiou S (2007) Probiotics for the treatment of women with bacterial vaginosis. Clin Microbiol Infect 13:657–664
Gibbs RS (2007) Asymptomatic bacterial vaginosis: is it time to treat? Am J Obstet Gynecol 196:495–496
Goldenberg RL, Culhane JF, Johnson DC (2005) Maternal infection and adverse fetal and neonatal outcomes. Clin Perinatol 32:523–529
Gould GW, Leistner L (2005) Update on hurdle technology approaches to food preservation. In: Davidson PM, Sofos JN, Branen AL (eds) Antimicrobials in food, 3rd edn. CRC Press, Boca Raton, pp 621–630
Guihard G, Bénédetti H, Besnard M, Letellier L (1993) Phosphate efflux through the channels formed by colicins and phage T5 in Escherichia coli cells is responsible for the fall in cytoplasmic ATP. J Biol Chem 268:17775–17780
Haggerty CL, Hillier SL, Bass DC, Ness RB, PID Evaluation and Clinical Health Study Investigators (2004) Bacterial vaginosis and anaerobic bacteria are associated with endometritis. Clin Infect Dis 39:990–995
Hashemi FB, Ghassemi M, Roebuck KA, Spear GT (1999) Activation of human immunodeficiency virus type 1 expression by Gardnerella vaginalis. J Infect Dis 179:924–930
Hashemi FB, Ghassemi M, Faro S, Aroutcheva A, Spear GT (2000) Induction of human immunodeficiency virus type 1 expression by anaerobes associated with bacterial vaginosis. J Infect Dis 181:1574–1580
Herranz C, Chen Y, Chung HJ, Cintas LM, Hernandez PE, Montville TJ, Chikindas ML (2001) Enterocin P selectively dissipates the membrane potential of Enterococcus faecium T136. Appl Environ Microbiol 67:1689–1692
Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG 2nd, Rao AV, McNellis D, Regan JA, Carey JC, Klebanoff MA (1995) Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 333:1737–1742
Hsu S-T, Breukink E, de Kruiff B, Kaptein R, Bonvin AMJJ, van Nuland NAJ (2002) Mapping the targeted membrane pore formation mechanism by solution NMR: the Nisin Z and lipid II interaction in SDS micelles. Biochemistry 41:7670–7676
Hsu S-T, Breukink E, Bierbaum G, Sahl HG, de Kruiff B, Kaptein R, van Nuland NAJ, Bonvin AMJJ (2003) NMR study of mersacidin and lipid II interaction in dodecylphosphocholine micelles. Conformational changes are a key to antimicrobial activity. J Biol Chem 278:13110–13117
Liebetrau A, Rodloff AC, Behra-Miellet J, Dubreuil L (2003) In vitro activities of a new des-fluoro(6) quinolone, garenoxacin, against clinical anaerobic bacteria. Antimicrob Agents Chemother 47:3667–3671
Lin L, Song J, Kimber N, Shott S, Tangora J, Aroutcheva A, Mazees MB, Wells A, Cohen A, Faro S (1999) The role of bacterial vaginosis in infection after major gynecologic surgery. Infect Dis Obstet Gynecol 7:169–174
Lubbe MM, Botha PL, Chalkley LJ (1999) Comparative activity of eighteen antimicrobial agents against anaerobic bacteria isolated in South Africa. Eur J Clin Microbiol Infect Dis 18:46–54
Marx R, Stein T, Entian KD, Glaser SJ (2001) Structure of the Bacillus subtilis peptide antibiotic subtilosin A determined by 1H NMR and matrix assisted laser desorption/ionization time-of-flight mass spectrometry. J Protein Chem 20:501–506
Molenaar D, Abee T, Konings WN (1991) Continuous measurement of the cytoplasmic pH in Lactococcus lactis with a fluorescent pH indicator. Biochim Biophys Acta 1115:75–83
Nelson DB, Bellamy S, Nachamkin I, Ness RB, Macones GA, Allen-Taylor L (2007) First trimester bacterial vaginosis, individual microorganism levels, and risk of second trimester pregnancy loss among urban women. Fertil Steril 88:1396–1403
Newton ER, Piper J, Peairs W (1997) Bacterial vaginosis and intraamniotic infection. Am J Obstet Gynecol 176:672–677
Oakeshott P, Kerry S, Hay S, Hay P (2004) Bacterial vaginosis and preterm birth: a prospective community-based cohort study. Br J Gen Pract 54:119–122
Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, Serwadda D, Li C, Kiwanuka N, Hillier SL, Rabe L, Gaydos CA, Quinn TC, Konde-Lule J (1997) HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 350:546–550
Shelburne CE, An FY, Dholpe V, Ramamoorthy A, Lopatin DE, Lantz MS (2007) The spectrum of antimicrobial activity of the bacteriocin subtilosin A. J Antimicrob Chemother 59:297–300
Silkin L, Hamza S, Kaufman S, Cobb SL, Vederas JC (2008) Spermicidal bacteriocins: lacticin 3147 and subtilosin A. Bioorg Med Chem Lett 18:3103–3106
Sims PJ, Waggoner AS, Wang CH, Hoffman JF (1974) Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry 13:3315–3330
Srinivasan S, Fredericks DN (2008) The human vaginal bacterial biota and bacterial vaginosis. Interdiscip Perspect Infect Dis 2008:750479
Stein T, Düsterhus S, Stroh A, Entian KD (2004) Subtilosin production by two Bacillus subtilis subspecies and variance of the sbo-alb cluster. Appl Environ Microbiol 70:2349–2353
Sutyak KE, Wirawan RE, Aroutcheva AA, Chikindas ML (2007) Isolation of the Bacillus subtilis antimicrobial peptide from the dairy product-derived Bacillus amyloliquefaciens. J Appl Microbiol 104:1067–1074
Sutyak KE, Anderson RA, Dover SE, Feathergill KA, Aroutcheva AA, Faro S, Chikindas ML (2008) Spermicidal activity of the safe natural antimicrobial peptide subtilosin. Infect Dis Obstet Gynecol 2008:540758
Taha TE, Gray RH, Kumwenda NI, Hoover DR, Mtimavalye LA, Liomba GN, Chiphangwi JD, Dallabetta GA, Miotti PG (1999) HIV infection and disturbances of vaginal flora during pregnancy. J Acquir Immune Defic Syndr Hum Retrovirol 20:52–59
Thennarasu S, Lee D-K, Poon A, Kawulka KE, Vederas JC, Ramamoorthy A (2005) Membrane permeabilization, orientation, and antimicrobial mechanism of subtilosin A. Chem Phys Lipids 137:38–51
Turvoskiy Y, Ludescher RD, Aroutcheva AA, Faro S, Chikindas ML (2009) Lactocin 160, a bacteriocin produced by vaginal Lactobacillus rhamnosus, targets cytoplasmic membranes of the vaginal pathogen, Gardnerella vaginalis. Probiotics Antimicrob Proteins 1:67–74
Weir E (2004) Bacterial vaginosis: more questions than answers. Can Med Assoc J 171:448
Winkowski K, Bruno MEC, Montville TJ (1994) Correlation of bioenergetic parameters with cell death in Listeria monocytogenes cells exposed to nisin. Appl Envrion Microbiol 60:4186–4188
Acknowledgments
This research was sponsored by the NIH NIAID Grant 1R01AI084137 “Multiplex Nanocarrier-Based Hydrogels for Prevention of Vaginal HIV Transmission, Highly Innovative Tactics to Interrupt Transmission of HIV (HIT-IT)” and the Rutgers University Life Science Commercialization Fund “Antimicrobial peptide subtilosin for control of bacterial vaginosis and feminine health care” (2008-2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sutyak Noll, K., Sinko, P.J. & Chikindas, M.L. Elucidation of the Molecular Mechanisms of Action of the Natural Antimicrobial Peptide Subtilosin Against the Bacterial Vaginosis-associated Pathogen Gardnerella vaginalis . Probiotics & Antimicro. Prot. 3, 41–47 (2011). https://doi.org/10.1007/s12602-010-9061-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-010-9061-4