Abstract
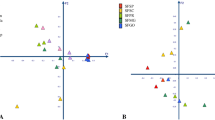
Helicoverpa armigera (Hübner), a lepidopteron pest, causes enormous crop loss in India and elsewhere in the world in part because of resistance to various insecticides. The major mechanism of insecticide resistance is metabolic, genetic and behavioural modification. In the present investigation, we analysed the possibility of the involvement of gut symbiotic bacteria in insecticide resistance. Isolation of culturable bacteria from insecticide-resistant and -susceptible populations of H. armigera collected from crops in India resulted in 6 different species of bacteria isolated from resistant populations and only one from the susceptible laboratory-reared population. PCR-denaturing gradient gel electrophoresis (DGGE) was used to amplify the partial gene sequences of the 16S rRNA–V3 region. The banding pattern was analysed by using PAST software. Jaccard’s multivariate cluster analysis revealed that the resistant populations formed a group as did the susceptible population. The Raup and Crick bacterial similarity index showed that the resistant populations were similar in bacterial composition regardless of host plant. Gut microbiota of the resistant populations had the highest diversity index, followed by the moderately resistant and susceptible populations. Microbiota of the highly resistant population from Amreli (Gujarat State) had the highest diversity index, 2.30 (Shannon H). Six bacterial species were isolated from H. armigera larvae by culture. Four bacterial species had a long-term and mutual association with the H. armigera populations and some of the microflora associated with insecticide resistant populations differ quantitatively from insecticide-susceptible populations.




Similar content being viewed by others
References
Behar, A., & Yuval, B. J. (2008). Gut bacterial communities in the Mediterranean fruit fly (Ceratitis capitata) and their impact on host longevity. Journal of Insect Physiology, 54, 1377–1383.
Boon, N., DeWindlt, W., Verstraete, W. W., & Top, E. M. (2002). Evaluation of nested PCR-DGGE with group-specific 16S rRNA primers for the analysis of Bacterial communities from different wastewater treatment plants. FEMS Microbiology Ecology, 39, 101–112.
Brennan, Y., Callen, W. N., Christoffersen, L., Dupree, P., Goubet, F., Healey, S., Hernandez, M., Keller, M., Li, K., Palackal, N., Sittenfeld, A., Tamayo, G., Wells, S., Hazlewood, G. P., Mathur, E. J., Short, J. M., Robertson, D. E., & Steer, B. A. (2004). Unusual microbial xylanases from insect guts. Applied and Environmental Microbiology, 70, 3609–3617.
Brownlie, J. C., & Johnson, K. N. (2009). Symbiont-mediated protection in insect hosts. Trends in Microbiology, 17(8), 348–354.
Brucker, R. M., & Bordenstein, S. T. (2012). Speciation by symbiosis. Trends in Ecology & Evolution, 27(8), 348–354.
Buchner, P. (1965). Endosymbiosis of animals with plant microorganisms. New York: Interscience. 909 pp.
Bunge, J., Woodard, L., Bohning, D., Foster, J. A., Connolly, S., & Allen, H. K. (2012). Estimating population diversity with CatchAll. Bioinformatic, 28(7), 1045–1047.
Chao, A. (1984). Non-parametric estimation of the number of classes in a population. Scandinavian Journal of Statistics, 11, 265–270.
Cummings, S. P., & Bambrough, L. (2008). The structure and stability of microbial communities. In T. V. Dijk (Ed.), Microbial ecology research trends (pp. 98–203). New York: Nova Science Publisher Inc.
Dar, S. A., Yao, L., Dongen, U., Kuenen, J. G., & Muyzer, G. (2007). Analysis of diversity and activity of Sulfate-reducing bacterial communities in sulfidogenic bioreactors using 16S rRNA and dsrB genes as molecular markers. Applied and Environmental Microbiology, 73, 564–604.
Davies, C. E., Hill, K. E., Wilson, M. J., Stephens, C. P., Hill, M., Harding, K. G., & Thomas, D. W. (2004). Use of 16S ribosomal DNA PCR and denaturing gradient gel electrophoresis for analysis of the microflora of healing and nonhealing chronic venous leg ulcers. Journal of Clinical Microbiology, 42(8), 3549–3557. doi:10.1128/JCM.42.8.3549-3557.
DeBary, A. (1879). Die Erscheinung der Symbiose. Strassburg: Verlag Von Karl J. Trubner.
Dillon, R. J., & Dillon, V. M. (2004). The gut bacteria of insects: non -pathogenic Interactions. Annual Review of Entomology, 49, 71–92.
Douglas, A. E. (1998). Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annual Review of Entomology, 43, 17–37.
Douglas, A. E. (2011). Lessons from studying insect symbioses. Cell Host Microbiology, 10(20), 359–368.
Finney, D. J. (1971). Probit analysis (3rd ed.). London: Cambridge University Press.
Gavriel, S., Jurkevitch, E., Gazit, Y., & Yuval, B. (2010). Bacterially enriched diet improves sexual performance of sterile male Mediterranean fruit flies. Journal of Applied Entomology. doi:10.1111/j.1439-0418.2010.01605.x.
Hosokawa, T., Kikuchi, Y., Nikoh, N., Shimada, M., & Fukastu, T. (2006). Strict host-symbionts co-speciation and reductive genome evolution in the insect gut bacteria. PLoS Biology, 4, e337.
Hui, X., Li, M., Yong, Z., Zhao, L., Zhang, L. H., & Yongping, H. (2007). Bacterial community in midguts of the silkworm larvae estimated by PCR/DGGE and 16S rDNA gene library analysis. Acta Entomologica Sinica, 50(3), 222–233.
Indiragandhi, P., Anandham, R., Madhaiyan, M., Poonguzhali, S., Kim, G. H., Saravanan, V. S., & Sa, T. (2007). Cultivable bacteria associated with larval gut of prothiofos-resistant, prothiofos-susceptible and field-caught populations of diamondback moth, Plutella xylostella and their potential for, antagonism towards entomopathogenic fungi and host insect nutrition. Journal of Applied Microbiology, 103, 2664–2675.
Kikuchi, Y., Hayatsu, M., Hosokawa, T., Nagayama, A., Tago, K., & Fukatsu, T. (2012). Symbiont-mediated insecticide resistance. Proceedings of the National Academy of Sciences, 109(22), 8618–8622.
Kim, Y. J., Lee, Y. J., Kim, G. H., Lee, S. W., & Ahn, Y. J. (1999). Toxicity of tebufenpyrad to Tetranychus urticae (Acari: Tetranychidae) and Amblyseius womersleyi (Acari: Phytoseiidae) under laboratory and field conditions. Journal of Economic Entomology, 92, 187–192.
Kim, J. Y., Lee, J., Shin, N. R., Yun, J. H., Whon, T. W., Kim, M. S., Jung, M. L., Roh, S. W., Hyun, D. W., & Bae, J. W. (2013). Orbus sasakiae sp. nov., a bacterium isolated from the gut of the butterfly Sasakia charonda, and emended description of the genus Orbus. International Journal of Systematic and Evolutionary Microbiology, 63, 1766–1770. doi:10.1099/ijs.0.041871-0.
Kirk, J. L., Beaudette, L. A., Hart, M., Moutoglis, P., Khironomos, J. N., Lee, H., & Trevors, J. T. (2004) Methods of studying soil microbial diversity. Journal of Microbiological Methods, 58, 169–188.
Kranthi, K.R. (2005). Insectcide resistance: Monitoring mechanism and management manual. CICR technical Bulletin, 143:155pp. ICAR. www.cicr.org.in/pdf/insecticide_resistance_KRK.pd.
Madhusudan, S., Jalali, S. K., Venkatesan, T., Lalitha, Y., & PrasannaSrinivas, R. (2011). 16SrRNA gene based identification of gut bacteria from laboratory and wild larvae of Helicoverpa armigera (Lepidoptera: Noctuidae) from tomato farm. The Bioscan, 6(2), 175–183.
Malhotra, J., Ankita, D., Saxena, A., Sangwan, N., Mukherjee, U., Pandey, N., Rajagopal, R., Khurana, P., Jitendra, P., Khurana, M., & Lala, R. (2012). Genome sequence of Acinetobacter sp. Isolated from the gut of the Polyphagous insect pest Helicoverpa armigera strain HA. Journal of Bacteriology, 194(18), 51–56. doi:10.1128/JB.01194-12.
Mishra, P. K., & Tandon, S. M. (2003). Gut bacterial flora of Helicoverpa armigera (Hub.) (Lepidoptera: Noctuidae). Indian Journal of Microbiology, 43(1), 55–56.
Morrison, M., Pope, P. B., Denman, S. E., & Mcsweeney, C. S. (2009). Plant biomass degradation by gut microbiomes: more of the same or something new? Current Opinion in Biotechnology, 20, 358–363.
Muyzer, G., De Waal, E. C., & Uitterlinden, A. G. (1993). Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Applied and Environmental Microbiology, 59, 695–700.
Muyzer, G. ( 1999). DGGE/TGGE a method for identifying genes from natural ecosystems. Current Opinion in Microbiology, 2, 317–322.
Nagarkatti, S., & Satyaprakash, R. (1974). Rearing Heliothis armigera Hub. On artificial diet. CIBC Technical Bulletin, 17, 169–173.
Nakatsu, C. H., Torsvik, V., & Ovreas, L. (2000). Soil community analysis using DGGE of 16S rDNA polymerase chain reaction products. Soil Science Society of America Journal, 64, 1382–1388.
O’Callaghan, M., Gerard, E. M., Heilig, G. H. J., Zhang, H., Jackson, T. A., & Glare, T. R. (2003). Denaturing gradient gel electrophoresis-a tool for plant protection research. New Zealand Plant Protection, 56, 143–150.
Ohkuma, M., & Kudo, T. (1996). Phylognetic diversity of the intestinal bacterial community in the termites Reticulitremes speratus. Applied and Environmental Microbiology, 62, 461–468.
Øvreas, L., & Torsvik, V. (1998). Microbial diversity and community structure in two different agricultural soil communities. Microbiology Ecology, 36, 303–315.
Paramasiva, I., Shouche, Y., Kulkarni, G. J., Krishnayya, P. K., Akbar, S. M., & Sharma, H. C. (2014a). Diversity in gut microflora of Helicoverpa armigera populations from different regions in relation to biological activity of Bacillus thuringiensis delta-endotoxin Cry1Ac. Archives of Insect Biochemistry and Physiology, 00(00), 1–13.
Paramasiva, I., Sharma, H. C., & Krishnayya, P. K. (2014b). Antibiotics influence the toxicity of the delta endotoxins of Bacillus thuringiensis towards the cotton bollworm, Helicoverpa armigera. BMC Microbiology, 14, 200–208.
Priya, G. N., Ojha, A., Mayur, K., Raj, A., & Rajagopal, R. (2012). Host plant induced variation in gut bacteria of Helicoverpa armigera. PLoS ONE, 7(1), 30768–30778.
Ratzka, C., Gross, R., & Feldhaar, H. (2012). Endosymbiont tolerance and control within insect hosts. Insects, 3, 553–572. doi:10.3390/insects3020553.
Raup, D., & Crick, R. E. (1979). Measurement of faunal similarity in paleontology. Journal of Paleontology, 53, 213–1227.
Reeson, A. F., Jankovic, T., Kasper, M. I., Rogers, S., & Austin, A. D. (2003). Applications of 16S rRNA-DGGE to examine the microbial ecology associated with a social wasp Vespuila germanica. Insect Molecular Biology, 12, 85–91.
Saraf, N., Makhija, S., & Kachole, M. (2015). Microbial diversity of proteolytic bacteria in the gut of Helicoverpa armigera. World Journal of Pharmaceutical Sciences, 3(5), 903–909.
Schabereiter, G. C., Lubitz, W., & Rolleke, S. (2003). Application of broad range 16S rRNA PCR amplification and DGGE fingerprinting for detection of tick-infecting bacteria. Journal of Microbiological Methods, 52, 251–260.
Sharon, G., Segal, D., Ringo, J. M., Hefetz, A., Rosenberg, I. Z., et al. (2010). Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proceedings of the National Academy of Sciences, 107(46), 20051–20056.
Shinde, A. A., Shaikh, F. K., Padul, M. V., & Kachole, M. S. (2012). Bacillus subtillis RTSBA6 6.00, a new strain isolated from gut of Helicoverpa armigera (Lepidoptera: Noctuidae) produces chymotrypsin-like proteases. Saudi Journal of Biological Sciences, 19, 317–323.
Smalla, K., Miruna, O. S., Milling, A., Heuer, H., Baumgarte, S., Becker, R., Neuber, G., Siegfried, K., Ulrich, A., Christopher, C., & Tebbe, S. (2007). Bacterial diversity of soils assessed by DGGE, T-RFLP and SSCP fingerprints of PCR-amplified 16S rRNA gene fragments: DO the different methods provide similar results? Journal of Microbiological Methods, 69, 470–479.
Tang, X., Freitak, D., Voge, H., Ping, L., Shao, Y., Corderoe, K., Andersen, G., Westermann, M., Heckel, D. G., & Boland, W. (2012). Complexity and variability of gut commensal microbiota in Polyphagous Lepidopteran Larvae. PLoS ONE, 7(7), e36978. doi:10.1371/journal.pone.0036978.
Tay, W. T., Soria, M. F., Walsh, T., Thomazoni, D., & Silvie, P. (2013). A brave new world for an old world pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. PLoS ONE, 8(11), 134–142. doi:10.137/journal.pone.0080134.
Torsvik, V., Daae, F., Sandaa, R. A., & Ovreas, L. (1998). Novel techniques for analysing microbial diversity in natural and perturbed environments. Journal of Biotechnology, 64, 53–62.
Werren, J.H. (2012). Symbionts provide pesticide detoxification. Proceedings of the National Academy of Sciences, 109 (22), 8364–8365. www.pnas.org/cgi/doi/10.1073/pnas.1206194109.
Xia, X., Zheng, D., Zhong, H., Qin, B., Gurrl, G., Vasseur, M., Lin, H., Bai, J., He, W., & You, M. (2012). DNA sequencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLoS ONE, 8(7), e68852. doi:10.1371/journal.pone.0068852.
Xiang, H., Gui-Fang, W., Jia, S., Huang, J., Miao, X. X., Zhou, Z., Zhao, L. P., & Dhuang, Y. P. (2006). Microbial communities in the larval midgut of laboratory and field populations of cotton bollworm Helicoverpa armigera. Canadian Journal of Microbiology, 52, 1080–1092.
Acknowledgments
The first author thanks Dr. N.K. Krishna Kumar, DDG (Horticulture), ICAR, New Delhi and Dr. Abraham Varghese, Director, NBAIR, Bangalore, for granting study leave to do her Doctoral programme at the University of Agricultural Sciences, Bengaluru, India. The work is part of the PhD work of the first author. The first author thanks Tagros Chemical India, Bayer Crop Science India, and Dow Agrochemicals for providing technical-grade insecticides to conduct the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gracy, R.G., Malathi, V.M., Jalali, S.K. et al. Variation in larval gut bacteria between insecticide-resistant and -susceptible populations of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Phytoparasitica 44, 477–490 (2016). https://doi.org/10.1007/s12600-016-0547-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-016-0547-9