Abstract
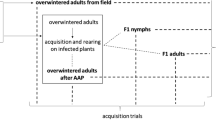
High population levels of Osbornellus horvathi (Matsumura 1908) in Mediterranean natural environments and our previous findings of adults infected by ‘Candidatus Phytoplasma phoenicium’, in Sicily in 2012, highlight the potential role of this species as a phytoplasma vector. In the present work, O. horvathi and other Auchenorrhyncha species were collected using sticky traps between May-October of 2013 in a natural area in Sicily. The insects were analyzed for phytoplasma detection and characterization. Among the 20 Auchenorrhyncha species collected, only O. horvathi was positive for phytoplasmas. Phytoplasma transmission to periwinkle and broad bean plants by field-collected phytoplasma-infected O. horvathi individuals was also carried out. The number and composition of Auchenorrhyncha populations in the area were described together with information on the biology of O. horvathi. ‘Candidatus Phytoplasma asteris’ was successfully transmitted to broad bean and periwinkle by field-collected ‘Ca. P. asteris’ and ‘Ca. P. phoenicium’ double-infected O. horvathi, thus highlighting its vector competence for the former phytoplasma. Further studies are required to assess the ability of O. horvathi to transmit ‘Ca. P. phoenicium’.



Similar content being viewed by others
References
Abou-Jawdah, Y., Sobh, H., & Akkary, M. (2009). First report of Almond witches’-broom phytoplasma (‘Candidatus Phytoplasma phoenicium’) causing a severe disease on nectarine and peach trees in Lebanon. EPPO Bulletin, 39, 94–98.
Abou-Jawdah, Y., Abdel Sater, A., Jawhari, M., Sobh, H., Abdul-Nour, H., Bianco, P. A., et al. (2014). Asymmetrasca decedens (Cicadellidae, Typhlocybinae), a natural vector of ‘Candidatus Phytoplasma phoenicium’. Annals of Applied Biology, 165, 395–403.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
Bagadia, P. G., Polashock, J., Bottner-Parker, K. D., Zhao, Y., Davis, R. E., & Lee, I. M. (2013). Characterization and molecular differentiation of 16SrI-E phytoplasmas associated with blueberry stunt disease in New Jersey. Molecular and Cellular Probes, 27(2), 90–97.
Bertaccini, A., Duduk, B., Paltrinieri, S., & Contaldo, N. (2014). Phytoplasmas and phytoplasma diseases: a severe threat to agriculture. American Journal of Plant Sciences, 5, 1763–1788.
Bertin, S., & Bosco, D. (2013). Insect vector transmission assays. In M. Dickinson & J. Hodgetts (Eds.), Phytoplasma: Methods and protocols, methods in molecular biology (pp. 87–108). New York: Humana Press.
Bosco, D., & D’Amelio, R. (2010). Transmission specificity and competition of multiple phytoplasmas in the insect vector. In P. G. Weintraub & P. Jones (Eds.), Phytoplasmas: Genomes, plant hosts and vectors (pp. 293–308). UK: MPG Books Group.
Bosco, D., & Tedeschi, R. (2013). Insect vector transmission assays. In M. Dickinson & J. Hodgetts (Eds.), Phytoplasma: Methods and protocols, methods in molecular biology (pp. 73–85). New York: Humana Press.
Casati, P., Quaglino, F., Abou-Jawdah, Y., Picciau, L., Cominetti, A., Tedeschi, R., et al. (2016). Wild plants could play a role in the spread of diseases associated with phytoplasmas of Pigeon pea witches’broom group (16SrIX). Journal of Plant Pathology, 98(1), 71–81.
D’Urso, V. (1995). Homoptera Auchenorrhyncha. In A. Minelli, S. Ruffo, & S. La Porta (Eds.), Checklist delle specie della Fauna Italiana 42 (pp. 1–35). Italy: Calderini.
D’Urso, V. & Alma, A. (2006). Insecta Homoptera Auchenorrhyncha (partim). In S. Ruffo & F. Stoch (Ed.), Checklist and distribution of the Italian fauna, Memorie del Museo Civico di Storia Naturale di Verona, 2. Serie, Sezione Scienze della Vita 17 with data on CD-ROM (pp. 155-157).
Dakhil, H., Hammad, E. A., El-Mohtar, C., & Abou-Jawdah, Y. (2011). Survey of leafhopper species in almond orchards infected with almond witches’-broom phytoplasma in Lebanon. Journal of Insect Science, 11, 1–12.
Danet, J. L., Balakishiyeva, G., Cimerman, A., Sauvion, N., Marie-Jeanne, V., Labonne, G., et al. (2011). Multilocus sequence analysis reveals the genetic diversity of European fruit tree phytoplasmas and supports the existence of inter-species recombination. Microbiology, 157, 438–450.
Davis, R. E., & Sinclair, W. A. (1998). Phytoplasma identity and disease etiology. Phytopathology, 88, 1372–1376.
Davis, R. E., Dally, E., Zhao, Y., Lee, I. M., Jomantiene, R., Detweiler, A. J., et al. (2010). First Report of a New Subgroup 16SrIX-E (‘Candidatus Phytoplasma phoenicium’-Related) Phytoplasma Associated with Juniper Witches Broom Disease in Oregon, USA. Plant Pathology, 59, 1161.
Deng, S., & Hiruki, C. (1991). Genetic relatedness between two nonculturable mycoplasmalike organisms revealed by nucleic acid hybridization and polymerase chain reaction. Phytopathology, 81, 1475–9.
Doyle, J. J., & Doyle, J. L. (1990). Isolation of plant DNA from fresh tissue. Focus, 12, 13–15.
D'Urso, V. (1995). Contributo alla conoscenza della distribuzione in Italia di alcune specie di Auchenorrinchi (Insecta Rhynchota: Homoptera). Naturalista Siciliano, Palermo, 19(1-2), 99–104.
Hermoso De Mendoza, A., Del Estal, P., Alcazar, M. D., Pérez-Otero, R., & Mansilla, P. (2012). Diferenciación entre Scaphoideus titanus Ball, vector de la Flavescencia Dorada de la vid, y una especie próxima, Osbornellus horvathi (Matsumura), recientemente encontrada en España (Hemiptera, Cicadellidae). Boletín de sanidad vegetal. Plagas, 38, 349–352.
Hoch, H. (2004). Hemiptera: Fulgomorpha, Cicadomorpha. In H. Hoch (Ed.), Fauna Europaea version 1.1, http://www.faunaeur.org (viewed February 26, 2007).
Hogenhout, S. A., Oshima, K., Ammar, E. D., Kakizawa, S., Kingdom, H. N., & Namba, S. (2008). Phytoplasmas: bacteria that manipulate plants and insects. Molecular Plant Pathology, 9, 403–423.
IRPCM. (2004). ‘Candidatus phytoplasma’, a taxon for the wall-less, non-helical prokaryotes that colonise plant phloem and insects. International Journal of Systematic and Evolutionary Microbiology, 54, 1243–1255.
Kenyon, L., Harrison, N. A., Ashburner, G. R., Boa, E. R., & Richardson, P. A. (1998). Detection of a pigeon pea witches’-broom-related phytoplasma in trees of Gliricidia sepium affected by little-leaf disease in Central America. Plant Pathology, 47, 671–680.
Khan, A. J., Al-Subhi, A. M., Calari, A., Al-Saady, N. A., & Bertaccini, A. (2007). A new phytoplasma associated with witches’ broom of Cassia italica in Oman. Bulletin of Insectology, 60, 269–270.
Lee, I. M., Hammond, R. W., Davis, R. E., & Gundersen, D. E. (1993). Universal amplification and analysis of pathogen 16S rDNA for classification and identification of mycoplasmalike organisms. Phytopathology, 83, 834–842.
Lee, I. M., Gundersen, D. E., Hammond, R. W., & Davis, R. E. (1994). Use of Mycoplasmalike Organism (MLO) Group-Specific oligonucleotide primers for nested-PCR assays to detect mixed-MLO infections in a single host plant. Phytopathology, 84, 559–566.
Lee, I. M., Gundersen-Rindal, D. E., Davis, R. E., & Bartoszyk, I. M. (1998a). Revised classification scheme of phytoplasmas based on RFLP analyses of 16S rRNA and ribosomal protein gene sequences. International Journal of Systematic Bacteriology, 48(4), 1153–1169.
Lee, I. M., Gundersen-Rindal, D. E., & Bertaccini, A. (1998b). Phytoplasma: ecology and genomic diversity. Phytopathology, 88(12), 1359–1366.
Lee, I. M., Davis, R., & Gundersen-Rindal, D. (2000). Phytoplasma: phytopathogenic mollicutes. Annual Reviews of Microbiology, 54, 221–255.
Lee, I. M., Gundersen-Rindal, D. E., Davis, R. E., Bottner, K. D., Marcone, C., & Seemüller, E. (2004). ‘Candidatus Phytoplasma asteris’, a novel phytoplasma taxon associated with aster yellows and related diseases. International Journal of Systematic and Evolutionary Microbiology, 54(4), 1037–1048.
Lee, I. M., Bottner-Parker, K. D., Zhao, Y., Davis, R. E., & Harrison, N. A. (2010). Phylogenetic analysis and delineation of phytoplasmas based on secY gene sequences. International Journal of Systematic and Evolutionary Microbiology, 60, 2887–2897.
Marcone, C. (2014). Molecular biology and pathogenicity of phytoplasmas. Annals of Applied Biology, 165, 199–221.
Marcone, C., Ragozzino, A., Camele, I., Rana, G. L., & Seemüller, E. (2001). Updating and extending genetic characterization and classification of phytoplasmas from wild and cultivated plants in Southern Italy. Journal of Plant Pathology, 83, 133–138.
Marzachì, C., Verati, F., & Bosco, D. (1998). Direct PCR detection of phytoplasmas in experimentally infected insects. Annals of Applied Biology, 133, 45–54.
Marzachì, C., Milne, R. G., Bosco, D., & Pandalai, S. G. (2004). Phytoplasma–plant–vector relationships. Recent Research Developments in Plant Pathology, 3, 211–241.
Matsumura, S. (1908). Neue Cicadinen aus Europa und Mittelmeergebiet. Journal of the College of Science, Imperial University of Tokyo, 23(6), 1–46.
Molino Lova, M., Quaglino, F., Abou-Jawdah, Y., Choueiri, E., Sobh, H., Casati, P., et al. (2011). Identification of New 16SrIX Subgroups, -F and -G, among ‘Candidatus Phytoplasma phoenicium’ Strains Infecting Almond, Peach and Nectarine in Lebanon. Phytopathologia Mediterranea, 50, 273–282.
Ossiannilsson, F. (1978). The Auchenorrhynca (Homoptera) of Fennoscandia and Denmark. Part 1: Introdution, infraorder Fulgoromorpha. Fauna Entomologica Scandinavica, 7(1), 1–222.
Ossiannilsson, F. (1981). The Auchenorrhynca (Homoptera) of Fennoscandia and Denmark. Part 2: the families Cicadidae, Cercopidae, Membracidae and Cicadellidae (exl. Deltocephalinae). Fauna Entomologica Scandinavica, 7(2), 223–593.
Ossiannilsson, F. (1983). The Auchenorrhynca (Homoptera) of Fennoscandia and Denmark. Part 3: the family Cicadellidae: Deltocephalinae, Catalogue. Literature and Index. Fauna Entomologica Scandinavica, 7(3), 594–978.
Quaglino, F., Kube, M., Jawhari, M., Abou-Jawdah, Y., Siewert, C., Choueiri, E., et al. (2015). ‘Candidatus Phytoplasma phoenicium’ associated with almond witches’-broom disease: from draft genome to genetic diversity among strain populations. BMC Microbiology, 15, 148.
Rashidi, M., D’Amelio, R., Galetto, L., Marzachì, C., & Bosco, D. (2014). Interactive transmission of two phytoplasmas by the vector insect. Annals of Applied Biology, 165, 404–413.
Rizza, S., D’Urso, V., Marzachì, C., & Tessitori, M. (2013). Osbornellus horvathi potential vector of 16SrIX phytoplasmas. Petria, 23(1), 91–94.
Salehi, M., Izadpanah, K., & Siampour, M. (2007). Characterization of a phytoplasma associated with cabbage yellows in Iran. Plant Disease, 91, 625–630.
Saracco, P., Bosco, D., Veratti, F., & Marzachì, C. (2006). Quantification over time of chrysanthemum yellows phytoplasma (16Sr-I) in leaves and roots of the host plant Chrisanthemum carinatum (Schousboe) following inoculation with its insects vector. Physiological and Molecular Plant Pathology, 67, 212–219.
Seemüller, E., Marcone, C., Lauer, U., Ragozzino, A., & Göschl, M. (1998). Current status of molecular classification of the phytoplasmas. Journal of Plant Pathology, 80, 3–26.
Spallino, R. E., Rizza, S., Marzachì, C., & Tessitori, M. (2013). Spartium witches’broom in Sicily. Petria, 23(1), 41–44.
Tedeschi, R., Picciau, L., Quaglino, F., Abou-Jawdah, Y., Molino Lova, M., Jawhari, M., et al. (2015). A cixiid survey for natural potential vectors of ‘Candidatus Phytoplasma phoenicium’ in Lebanon and preliminary transmission trials. Annals of Applied Biology, 166, 372–88.
Verdin, E., Salar, P., Danet, J. L., Choueiri, E., Jreijiri, F., El Zammar, S., et al. (2003). ‘Candidatus Phytoplasma phoenicium’, a new phytoplasma associated with an emerging lethal disease of almond trees in Lebanon and Iran. International Journal of Systematic and Evolutionary Microbiology, 53, 833–838.
Wei, W., Davis, R. E., Lee, I. M., & ZHAO, Y. (2007). Computer simulated RFLP analysis of 16S rRNA genes: identification of ten new phytoplasma groups. International Journal of Systematic and Evolutionary Microbiology, 57, 1855–1867.
Weintraub, P. G., & Beanland, L. (2006). Insect vectors of phytoplasmas. Annual Review of Entomology, 51, 91–111.
Zahniser, J. N., & Dietrich, C. H. (2010). Phylogeny of the leafhopper subfamily Deltocephalinae (Hemiptera: Cicadellidae) based on molecular and morphological data with a revised family-group classification. Systematic Entomology, 35, 489–511.
Zhao, Y., Wei, W., Lee, I. M., Shao, J., Suo, X., & Davis, R. E. (2009). Construction of an interactive online phytoplasma classification tool, iPhy Classifier, and its application in analysis of the peach X-disease phytoplasmas group (16SrIII). International Journal of Sistematic and Evolutionary Microbiology, 59, 2582–2593.
Acknowledgments
This research was funded by the Regione Sicilia (Assessorato Regionale delle Risorse Agricole e Alimentari, Servizio 5 Fitosanitario Regionale), within the project ‘Individuazione di focolai del fitoplasma (16SrRNA V) agente causale della Flavescenza dorata della vite’. We would like to thank Prof. Domenico Bosco (DISAFA, University of Turin) for his critical reading of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s12600-016-0555-9.
Rights and permissions
About this article
Cite this article
Serena, R., Antonella, P., Vera, D. et al. Transmission of ‘Candidatus Phytoplasma asteris’ (16SrI) by Osbornellus horvathi (Matsumura 1908) co-infected with “Ca. Phytoplasma phoenicium” (16SrIX). Phytoparasitica 44, 491–500 (2016). https://doi.org/10.1007/s12600-016-0545-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-016-0545-y