Abstract
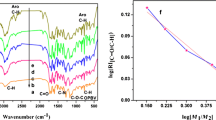
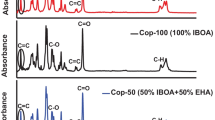
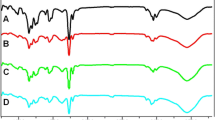
A new type of co-polymer between anthranilic acid cinnamoyl ester and monoethyleneglycol dimethacrylate was synthesized via aza-Michael addition polymerization reaction by bulk polymerization method without adding any catalyst. The polymerization reaction was carried out at 100 °C for 2 h under N2 atmosphere with mild stirring. The co-polymer was synthesized at various monomer ratios. The above-synthesized co-polymer was characterized by Fourier transform infrared (FTIR) spectroscopy, UV–visible reflectance spectroscopy, X-ray diffraction (XRD), thermogravimetric analysis (TGA), scanning electron microscopy, etc. The FTIR data confirmed the 0.032 order of reaction with respect to [M1/M2]. The amorphous nature of the above-synthesized polymer was confirmed by XRD. The non-isothermal degradation kinetics was followed at five different heating rates with five different models. The goal of the present work is to compare the results from TGA data and select the best approach for the synthesized polymer. The activation energy (Ea) values were also determined by model-free methods. The experimental results were carefully analysed and compared with the literature values.











Similar content being viewed by others
References
Gu Y, Barrault J, Jerome F (2008) Glycerol as an efficient promoting medium for organic reactions. Adv Synth Catal 350:2007–2012
Devatha P, Podgorski M, Chatani S, Gong T, Xi W, Fenoli CR, Bowman CN (2014) The thiol-Michael addition click reaction: a powerful and widely used tool in materials chemistry. Chem Mater 26:724–744
Tamami B, Fadavi A, Tamami M (2006) Polyacrylamide supported phenolate as a heterogeneous, efficient, recyclable and selective catalyst for aza-and thio-Michael addition in aqueous media. Iran Polym J 15:789–807
Mather BD, Viswanathan K, Millar KM, Long TE (2006) Michael addition reactions in macromolecular design for emerging technologies. Prog Polym Sci 31:487–531
Shen Y, Ma Y, Li Z (2013) Facile synthesis of dendrimers combining aza-Michael addition with thiol-yne click chemistry. J Polym Sci Part A Polym Chem 51:708–715
Sundararaja G, Prabagaran N (2001) A new polymer anchored chiral catalyst for asymmetric Michael addition reactions. Org Lett 3:389–392
Espeel P, Goethals F, Driessen F, Nguyen LTH, Prez FED (2013) Additive free preparation of functionalized polyurethane via amine-thiol-ene conjugation. Polym Chem 9:2449–2457
Han SS, He WD, Li J, Li Y, Sun XL, Zhang BY, Pan TT (2009) Reducible polyethylenimine hydrogels with disulfide crosslinkers prepared by Michael addition chemistry as drug delivery carriers: synthesis, properties, and in vitro release. J Polym Sci Part A Polym Chem 47:4074–4082
Su HL, Hsu JM, Pan JP, Wang TH, Yu FE (2011) Kinetic and structural studies of the polymerization of N, N′-bismaleimide-4,4′-diphenylmethane with barbituric acid. Polym Eng Sci 53:1188–1197
Chatani S, Podgorski M, Wang C, Bowman CN (2014) Facile and efficient synthesis of dendrimers and one-pot preparation of dendritic linear polymer conjugates via a single chemistry: utilization of kinetically selective thiol-Michael addition reactions. Macromolecules 47:4894–4900
Yang L, Xu LW, Xia CG (2005) Highly efficient KF/Al2O3 catalyzed versatile hetero-Michael addition of nitrogen, oxygen, and sulfur nucleophiles to α, β-ethylenic compounds. Tetrahedron Lett 46:3279–3282
Malerich JP, Zhu Y, Rawal VH (2010) Squaramide catalyzed enantioselective Michael addition of diphenyl phosphite to nitroalkenes. Angew Chem Int Ed 122:157–160
Wabnitz TC, Yu JQ, Spencer JB (2004) Evidence that protons can be the active catalysts in Lewis acid mediated hetero-Michael addition reactions. Chem A Eur J 10:484–493
Fustero S, Jimenez D, Rosello MS, Pozo CD (2007) Microwave assisted Tandem cross metathesis intramolecular aza-Michael reaction: an easy entry to cyclic β-amino carbonyl derivatives. J Am Chem Soc 129:6700–6701
Vakulya B, Varga S, Soos T (2008) Epi-cinchona based thiourea organocatalyst family as an efficient asymmetric Michael addition promoter: enantioselective conjugate addition of nitroalkanes to chalcones and α, β-unsaturated N-acylpyrroles. J Org Chem 73:3475–3480
Pitchaimari G, Vijayakumar CT (2014) Functionalized N-(4-hydroxy phenyl) maleimide monomers: kinetics of thermal polymerization and degradation using model-free approach. J Appl Polym Sci 131:1–11
Rammo NN, Mahdi HAM, Al-Haitham Ibn (2013) Thermal degradation kinetics of polyamide 6,6 cable ties by thermogravimetric analysis. J Pure Appl Sci 26:199–210
Jabal AA, Tan IM, Man Z, Maitra S (2009) A kinetic analysis of the degradation of grafted anionic polyacrylamide gel under nonisothermal condition. J Eng Sci Tech 4:364–373
Aigbodion VS, Hassan SB, Atuanya CU (2012) Kinetics of isothermal degradation studies by thermogravimetric data: effect of orange peels ash on thermal properties of high density polyethylene (HDPE). J Mater Environ Sci 3:1027–1036
Turmanova SC, Genieva SD, Dimitrova AS, Vlaev LT (2008) Non-isothermal degradation kinetics of filled with rice husk ash polypropene composites. Express Polym Lett 2:133–146
Murichan N, Cherntongchai P (2014) Kinetic analysis of thermal degradation of polyolefin mixtures. Int J Chem Eng Appl 5:169–175
Khaghanikavkani E, Farid MM (2011) Thermal pyrolysis of polyethylene: kinetic study. Eng Sci Tech 2:1–10
Bauri K, Roy SG, Arora S, Dey RK, Goswami A, Madras G, De P (2013) Thermal degradation kinetics of thermoresponsive poly (N-isopropylacrylamide-co-N, N-dimethylacrylamide) copolymers prepared via RAFT polymerization. J Therm Anal Calorim 111:753–761
Chrissafis K (2009) Kinetics of thermal degradation of polymers. J Therm Anal Calorim 95:273–283
Goswami A, Srivastava G, Umarji AM, Madras G (2012) Thermal degradation kinetics of poly(trimethylol propane triacrylate)/poly(hexane diol diacrylate) interpenetrating polymer network. Thermochim Acta 547:53–61
Wu D, Liu Y, He C, Chung V, Goh S (2004) Effects of chemistries of trifunctional amines on mechanism of Michael addition polymerization with diacrylates. Macromolecules 37:6763–6770
Goswami S, Kiren K (2012) Application of Kissinger analysis to glass transition and study of thermal degradation kinetics of phenolic–acrylic IPNs. Bull Mater Sci 35:657–664
Zugates GT, Tedford NC, Zumbuehl A, Kang CS, Griffith LG, Langer R, Anderson DG (2007) Gene delivery properties and end modified poly(β-amino esters). Bioconjugate Chem 18:1887–1896
Vyazovkin SV (2001) Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem 22:178–183
Doyle CD (1961) Kinetic analysis of thermogravimetric data. J Appl Polym Sci 5:285–292
Parthasarathy V, Dhanalakshmi V, Anbarasan R (2015) Thermal studies on benzamide and benzanilide grafted LDPE. Therm Anal Calorim 119:73–84
Amala Jeya Ranchani A, Parthasarathy V, Anithadevi A, Meenarathi B, Anbarasan R (2017) Synthesis, characterization and catalytic activity of nanosized Ni complexed aminoclay. Appl Nanosci 7:577–588
Jancirani A, Kohila V, Meenarathi B, Anbarasan R (2016) Synthesis, characterisation and non-isothermal degradation kinetics of novel poly(mono ethylene glycoldimethacrylate-co-4-aminobenzoate). Bull Mater Sci 39:1725–1733
Sribala G, Meenarathi B, Anbarasan R (2017) Synthesis, characterization, and catalytic activity of fluorescent polyimide nanocomposites. J Appl Polym Sci 134:1–9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest among the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jancirani, A., Kohila, V., Meenarathi, B. et al. Non-isothermal degradation kinetics of novel poly(monoethyleneglycol dimethacrylate-co-anthranilic acid cinnamoyl ester) synthesized via aza-Michael addition polymerization reaction. Int J Plast Technol 23, 29–38 (2019). https://doi.org/10.1007/s12588-019-09231-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12588-019-09231-w