Abstract
Rodent thoracic veins are characterized by an extended myocardial coating. In the present study, the electrical activity in the cardiac tissue of the rat azygos vein (AZV) was investigated for the first time. The atrial-like action potentials (AP) and atrial-like conduction of the excitation were observed in the rat AZV under continuous electrical pacing. Termination of electrical pacing resulted in spontaneous positive shift of resting membrane potential (RMP) in AZV. Boradrenaline induced biphasic effects on RMP in all quiescent AZV preparations but only in 25% preparations—bursts of spontaneous AP, which were suppressed by both α- and β-adrenoreceptor antagonists. Phenylephrine induced additional depolarization of RMP in quiescent AZV preparations, while isoproterenol caused hyperpolarization. In conclusion, bioelectrical properties of the rat AZV resemble those of atrial myocardium under continuous electrical pacing; however, depolarized RMP and NA-induced spontaneous AP characterize AZV as a tissue prone to rare automaticity.
Similar content being viewed by others
Introduction
Thoracic veins contain a cardiac tissue in a form of “myocardial sleeves” which extend from the atria to the distal regions of the vessels [1, 2]. The cardiac tissue both in pulmonary and caval veins has been known for more a century in non-mammalian species, various mammals and human [3,4,5]. It has also been shown that a myocardial tissue in distinct regions of the thoracic veins is functionally active and demonstrates both electrical excitability and contractility [6, 7]. A number of studies have focused on the pulmonary vein (PV) myocardium electrophysiology, either in laboratory animals or human because this tissue is considered as a source of atrial fibrillation (AF). It is well established that PV myocardium acts as a source of AF due to various mechanisms, including ectopic automaticity and re-entrant conduction [8, 9]. Less attention has been paid to the cardiac tissue of the caval veins (CV); however, action potential and ectopic automaticity have been demonstrated in this tissue in several experimental investigations [10, 11]. caval veins-derived AF has also been described, similarly to PV [12, 13].
In addition, cardiomyocytes have been demonstrated in the wall of the azygos vein (AZV) in non-rodent and rodent mammals [14] which is a derivative of the superior (or anterior in quadruped animals) CV. The cardiac tissue manifests at least at the level of the AZV mouth in human [15]. The extensive myocardial sleeve has been revealed in the rat AZV both in proximal and distal vessel regions [16]. It has been shown that cardiac tissue in the rat AZV is characterized by the ability to contract in response to electrical stimuli delivery at a high rate [17]. Unlike the PV or the CV, the electrophysiological properties of the rat AZV have not yet been investigated.
Numerous autonomic nerves have been demonstrated in the junction region between atria and veins and also in the myocardial sleeves of thoracic veins [18, 19]. It has been established previously that electrical activity in canine, rabbit, guinea pig pulmonary and caval veins is highly dependent on autonomic nerves or adrenergic stimulation [20, 21]. It is widely accepted at the present time that thoracic veins arrhythmogenity is highly dependent on sympathetic or adrenergic stimulation [22]. Adrenaline application causes spontaneous action potentials in the rat PV myocardial sleeves [23]. Nevertheless, the effects of the adrenergic stimulation on the AZV myocardium have yet to be investigated.
Thus, the present study aims to characterize the bioelectrical activity in the rat AZV in basal conditions (i.e. without adrenergic stimulation) and under adrenergic stimulation. The AZV contains the most distant cardiac tissue from the atrial wall in adult mammalians. It is unclear whether the venous cardiac tissue is electrically coupled to the atrial myocardium or how it conducts excitation waves. Therefore, the ability of the AZV myocardium to conduct excitation waves was estimated in the current study.
Materials and methods
Animals
All experimental procedures were carried out in accordance with the Guide for the Care and the Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 2010) and approved by the Ethics Committee of the MSU Biological department. Male Wistar rats weighing 250–300 g (n = 57, 10 weeks old) were provided by the Scientific complex of biomedical technologies animal plant (Moscow region, Russia). Animals were held in the animal house for 2 weeks under a 12 h:12 h light:dark photoperiod in standard cages prior to the experiment and fed ad libitum.
Isolation of atria and azygos vein
Heparinized (100 IU/100 g, i.p.) rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.). The chest was opened, the left atria (LA), right atria (RA) or left superior vena cava (LSVC) with adjoined AZV were separated from surrounding fascia and fat, rapidly excised, incised and pinned with the endocardial side up to the bottom of a 5-ml perfusion chamber filled with physiological (Tyrode) solution of the following composition (in mM): NaCl 118.0, KCl 2.7, NaH2PO4 2.2, MgCl2 1.2, CaCl2 1.2, NaHCO3 25.0, glucose 11.0, pH 7.4 ± 0.2 bubbled by 95% O2 and 5% CO2 gas mixture. The constant perfusion with a flow rate of 15 ml/min at 37 °C was started immediately after preparation. Electrical pacing for rhythm maintenance was started immediately after the dissection. Tissue excitation was elicited by constant 2-ms pulses (with amplitude twice above the threshold) at a pacing rate of 3 Hz. A pair of silver electrodes used for the pacing was placed at the atria appendage or at the surface of the superior CV close to the adjoined AZV. In experiments with quiescent preparations, the AZV was separated from the CV.
Microelectrode recording
Resting membrane potential (RMP) and electrically evoked action potentials (AP) were recorded by glass microelectrodes (10–20 MΩ) filled with 3 M KCl. A Warner intracellular electrometer (IE-210) was used to amplify the signals. The AP were digitized at a 10-kHz sampling rate by using an analog–digital converter (E-154, ADC L-card; Russia) and analyzed using custom software (PowerGraph 3.3 Professional, v.3.3.8; Russia). Only a series of a stable impalements demonstrating AP overshooting and fast AP upstroke velocity were taken into account. Measurements were performed after 60 min of equilibration. The AP 50 and 90% durations (APD50 and APD90) and RMP level were calculated.
Optical mapping
The otical mapping setup consisted of a PDA (WuTech H-469V; Gaithersburg, MD, USA) designed for high speed data acquisition (1.63 Kfps). Macroscopic projections of the cardiac tissue preparations were transferred to the photodiodes array with the aid of the optical system including adapters and a Computar V5013 (CBC Group, Japan) camera lens (focal length 50 mm, aperture ratio 1:1.3) mounted at a distance of 24 mm from the tissue surface. The optical system allowed the projection of an area of 5 mm in diameter to the 464 photodiodes (each 0.75 mm in diameter) which were assembled in a hexagonal array with a physical aperture of 19 mm (22 photodiodes in the longest row). Thus, each photodiode covered a surface of 0.23 mm in diameter approximately.
It was possible to project the lens field of view to the monitoring CCD camera (NexImage, Celestron, USA) via a prism insertion included to the optical system. A CCD camera was used for the colocalization of the mapping area and preparation sites during the experiments and data analysis.
Excitation light was emitted by three self-made green LED (520 ± 40 nm) arrays surrounding the perfusion chamber. A long-pass emission filter (λ > 650 nm) was positioned in front of the camera lens.
Potential-sensitive dye di-4-ANEPPS (5 mg/ml in DMSO; Molecular Probes, Eugene, OR, USA) was added to the superfusion solution (5 μmol/l) and 20 min staining was carried out. The final concentration of DMSO in the solution was below 0.1%, which is acceptable for electrophysiological studies.
In all cases, fluorescent signals were recorded continuously for 5 s with 0.614-ms frame intervals, digitized using a data acquisition system (CardioPDA-III; RedShirtImaging, Decatur, GA, USA) and analyzed using Cardioplex (v.8.2.1, RedShirtImaging) software. The resting fluorescence was determined before each signal recording, and the obtained signals were normalized to the resting fluorescence. The signals were processed via a low-pass filter using a median algorithm and were normalized to the resting fluorescence. Also, a minimal high-pass filter was applied to remove long-time constant photodiode-derived basal drift. The maximum upstroke derivative (dF/dt max) for each optical AP was calculated to determine the activation times in the mapped areas. Isochronic activation maps were constructed from activation times using an in-house-developed software.
Drugs
Noradrenaline (NA), isoproterenol (ISO), phenylephrine (PHE), prazosin and propranolol (PPO) were purchased from SigmaAldrich (St Louis, MO, USA). Di-4-ANEPPS was purchased from Molecular Probes.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism v.6. Hypothesis testing was carried out using an ANOVA with further Tukey’s post hoc multiple comparisons test and paired t test. A p value < 0.05 was considered statistically significant. All results are expressed as mean ± SD for n experiments.
Results
The action potentials and resting membrane potential in the AZV under basal condition
The atrial-like AP with overshoot and rapid AP upstroke accompanied by stable RMP were observed in both proximal and distal regions (Fig. 1a) of the electrically paced AZV preparations (Fig. 1b).
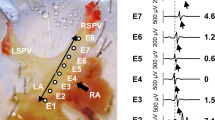
Electrically evoked action potentials in the rat AZV. a The points of AP recordings (dots) and sites (white frames) of excitation mapping in the proximal (1) and distal (2) portions of the AZV. b Representative examples of AP from the electrically paced left atria (LA), right atria (RA), proximal (AZV prox ) and distal (AZV dist ) regions of the AZV. AP duration at level of 50% (c) and 90% (d) repolarization (APD50, APD90, respectively) in LA, RA, proximal and distal portions of the AZV. LSVC left superior vena cava; *,# p < 0.05 (ANOVA); *all sites vs. RA; #proximal vs. distal azygos
The duration of AP was similar in the AZV and LA, in which APD50 and APD90 were 9.5 ± 4 and 31.5 ± 10 ms, respectively (n = 9). However, APD90 in both proximal (24.8 ± 3 ms, n = 9) and distal (40.4 ± 4 ms, n = 6) AZV regions were significantly (p < 0.0001, p = 0.013, respectively) shorter if compared to the RA in which APD90 was 57.1 ± 15 ms (n = 7). APD50 was significantly shorter in comparison to RA (18.8 ± 4 ms, n = 6, p = 0.0002) only in the proximal AZV region (6.2 ± 1 ms, n = 9). In addition, APD50 in proximal AZV was significantly shorter cmpared to the distal region (14.3 ± 2 ms, p = 0.0153, n = 6; Fig. 1c, d).
The resting membrane potential was similar in both proximal and distal regions (−71.2 ± 6 mV, n = 11) of the paced AZV; however, it was significantly positive in comparison to LA (−79.1 ± 4 mV, n = 6, p = 0.0195) and RA (−80.8 ± 4, n = 7, p = 0.0030) under basal conditions (Fig. 2a). Termination of the electrical pacing of AZV resulted in spontaneous and significant (p = 0.0002) positive drift of RMP with further stabilization at the level of −60.3 ± 8 mV (n = 11) (Fig. 2b, c). In contrast to the AZV, no substantial depolarization of RMP was observed in LA and RA after pacing termination. In addition, no spontaneous AP were observed in quiescent AZV among all preparations under basal conditions. The resumption of the electrical pacing resulted in the hyperpolarization of RMP and its stabilization at a level identical (−70.3 ± 7 mV, n = 11, p > 0.05) to those before a quiescence period.
Resting membrane potential (RMP) in electrically paced (a) and quiescent rat azygos vein (AZV). Termination of the electrical pacing causes spontaneous drift of membrane potential in quiescent AZV (b) in contrast to LA or RA: example tracks represent RMP changes under pacing maneuvers (the tracks are shown from the moment of pacing termination) (c). Resumption of the electrical pacing results in the hyperpolarization of RMP (d). LA left atrium, RA right atrium. Paced electrically paced AZV, quiescent AZV after 5 min of stabilization without electrical pacing. *p = 0.0195, AZV vs. LA; # p = 0.0030, AZV vs. RA (ANOVA); **p = 0.0002, paced vs. quiescent AZV (paired t test)
The conduction of excitation in the paced AZV under basal conditions
A steady-state continuous electrical pacing with cycle length 300 ms caused excitation of the proximal site of AZV and anterograde conduction of the excitation (i.e. from the LSVC–AZV junction region to the distal portion of the AZV). No conduction blocks or other abnormalities (Fig. 3) were observed in the AZV at least at a distance of 1 cm from the LSVC–AZV junction in all cases (n = 6) under continuous pacing. A similar pattern of anterograde conduction was observed in AZV if the excitation was initiated at the LSVC–AZV junction region or more distantly at the right atria. More distally than 1–1.2 cm from the LSVC–AZV junction, no optical AP were observed in all preparations (n = 6). As in the previous microelectrode experiments, no spontaneous excitations were observed in quiescent AZV preparations. Also, the AZV is characterized by stable beat-to-beat excitation (Fig. 3e, f).
Propagation of the excitation in the electrically paced AZV. The photographs (a, b) of the mapped area in two sites in the AZV (proximal and distal, also indicated as 1 and 2 in Fig. 1a) with superimposed optical signals (optical AP, red peaks) registered by individual photodiodes. Representative examples (c, d) of the isochronic maps from the proximal and distal regions of the AZV which demonstrate anterograde conduction of the excitation under electrical pacing. Blue color corresponds with earlier excitation; red color with later excitation. Horizontal arrows indicate general direction of the excitation propagation. Optical traces (e, f) of a three consecutive excitation cycles (cycle length 300 ms) from the points in AZV which are indicated in the isochronic maps (in c, d). No substantial beat-to-beat AP variability was observed under pacing with 300-ms cycle length. Note that isocronic maps were received from same tissue preparation but consecutively from the proximal and distal portions of the vein. AZV prox and AZV dist the proximal and distal regions of the AZV. CV left superior vena cava. Asterisks denote position of the pacing electrodes. Sloped arrows in (e) and (f) indicate motion artifacts (color figure online)
The electrical activity in the AZV under noradrenaline application
We tested the effects of 0.1–10 µmol/l noradrenaline (NA) on the bioelectrical properties of the quiescent AZV. While 0.1 µmol/l NA caused no changes in RMP, 1–10 µmol/l NA induced substantial biphasic RMP alteration in AZV preparations. The application of NA resulted in rapid RMP hyperpolarization which was followed by delayed depolarization (Fig. 4a). These effects were dose-dependent and partially opposite: 1 µmol/l NA induced weak (2.5 ± 2 mV) hyperpolarization (p = 0.0706), but significant depolarization (4.1 ± 2 mV, p = 0.0150, n = 6); 10 µmol/l NA, in contrast, induced more pronounced hyperpolarization (8.3 ± 5 mV, p = 0.0010) and weak RMP depolarization (by 4.9 ± 3 mV, p = 0.1051, n = 6; Fig. 4b).
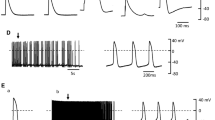
Effects of noradrenaline (NA) in the myocardial tissue of the azygos vein (AZV). Example track representing consequent RMP changes (transient hyperpolarization and delayed continuous depolarization) which occur in response to 10 µmol/l NA application in quiescent AZV (a). Alteration of RMP in AZV by 10 µmol/l noradrenaline (b). Representative example of the AP bursts (c) with initiating (d) and terminating action potentials (e) in AZV induced by NA later on NA-caused RMP depolarization. Control RMP in quiescent AZV under basal conditions, NA hyper and NA depol NA-induced hyperpolarization and depolarization, respectively, *p < 0.05 (control vs. NA hyper, control vs. NA depol, ANOVA)
The highest used NA concentration (10 µmol/l) caused spontaneous AP in the form of quasi-periodic bursts in 7 out of 28 (25%) quiescent AZV preparations (Fig. 4c). NA-induced firing was initiated at the phase of RMP depolarization 12 ± 8 min later at the beginning of catecholamine application. No spontaneous AP was observed under lower NA concentrations.
Parameters of NA-induced AP bursts varied greatly. The bursts were interposed by quiescent intervals, which varied from 43.1 to 202.9 s among the preparations. The total number of AP in a single burst varied from 25 to 123 and AP in bursts followed with a frequency of from 4.1 to 7.1 Hz. Nevertheless, membrane potential of the initial and terminating AP in bursts remained relatively stable: −58.5 ± 6 and −68.1 ± 9 mV, respectively. Also, RMP was characterized by a slow depolarization in the periods between the bursts. The delayed after-depolarization-like potentials that failed to reach threshold and evoke AP were observed at the end of bursts (Fig. 4e).
Effects of α- and β-adrenoreceptor antagonist on noradrenaline-induced firing in the AZVs preparations
Application of the α-adrenoreceptor antagonist prazosin (10 µmol/l) caused the cessation of NA-induced spontaneous activity in AZV in all experiments (n = 5). In addition, a significant RMP hyperpolarization (up to −85.5 ± 9 mV, p < 0.05) followed the prazosin-caused termination of the NA-firing (Fig. 5a). Similarly to prazosin, β-adrenoreceptor antagonist propranolol (PPO, 10 µmol/l) caused the termination of NA-induced firing in all cases (n = 5; Fig. 5a). However, no substantial RMP alteration was observed after PPO application and firing termination.
Effects of a selective α- and β-adrenoreceptor agonist in AZV preparations
The application of 0.1–1 µmol/l phenylephrine failed to alter resting membrane potential; however, 10 µmol/l PHE caused significant RMP depolarization (+ 10 ± 5 mV, n = 6, p = 0.0073; Fig. 6d) in quiescent AZV. A spontaneous firing in the form of periodic bursts was initiated by 10 µmol/l PHE only in one AZV preparation (1 out of 6); however, the pattern of PHE-induced firing was very close to that elicited by NA. In this case, the bursts were interposed by 8 ± 2 s quiescent intervals. The number and the frequency of AP in the bursts varied from 201 to 254 and from 3.9 to 5.6 Hz, respectively (Fig. 6a–c). The membrane potential of the firing triggering was −36.1 ± 2 mV, and the potential of the burst termination was −39.1 ± 2 mV.
Effects of α- and β-adrenoreceptors selective agonists on bioelectrical activity in the AZV. Examples of the phenylephrine-induced AP bursts in AZV (a) with initial (b) and terminal action potentials (c) demonstrated. Representative example of RMP depolarization caused by phenylephrine (d) and RMP hyperpolarization induced by ISO (e) in the quiescent AZV. f Alteration of RMP in quiescent AZV by adrenoreceptors agonists, *p < 0.05 (control vs. PHE, control vs. ISO, ANOVA)
In contrast to NA, the application of 1 and 10 µmol/l (but not 0.1 µmol/l) of ISO caused significant and rapid RMP hyperpolarization by 9.7 ± 4 mV (n = 6, p = 0.0019) and 8.3 ± 6 mV (n = 8, p = 0.0047), respectively, in quiescent AZV preparations (Fig. 6e, f). No spontaneous AP and firing was observed in the response to 0.1–10 µmol/l ISO administration.
Discussion
In the present study, the electrical properties of the rat AZV cardiac tissue were described for the first time. We have demonstrated atrial-like AP at two sites (more proximal and more distal) in the steady-state paced AZV preparations. The duration of the AP in proximal and distal regions of the rat AZV was similar to that in LA, but was shorter in comparison to the right atria. Also, the results of our experiments show that AP duration in the rat AZV demonstrates spatial variation.
At the present time, there are very few data available about AP duration in CV myocardium. The characteristics of AP in CV myocardium have been estimated only in dogs and rabbits. It has been demonstrated that AP in the canine superior caval vein (SVC) have a marked diversity of electrophysiological characteristics. It has been demonstrated that AP in canine SVC can be shorter as well as longer in comparison to the atrial tissue [24], with or without the pacemaker phenotype [11]. The features of an anterior caval vein AP are similar to those of atrial AP with stable resting membrane potential, rapid AP upstroke and high amplitude in rabbits [6]. Many of the previous studies were aimed at the characterization of the AP in pulmonary veins, which are other thoracic vessels that possess myocardial sleeves. It has been reported that AP duration in canine [25] and guinea pig [5, 26] PV myocardium is shorter compared with LA. In contrast, longer AP in the pulmonary vein in comparison with the LA has been shown in rabbits [27] and mice [28]. As for the rat, controversially longer [23], as well as shorter, AP [29] were observed in PV in comparison with the atrial myocardium. Therefore, it is difficult to determine a definite correlation between AP duration and cardiac tissue localization in thoracic veins due to significant variation between studies and a dependence on the animal species used. A local variations of the AP in the rat AZV are probably caused by regional differences in depolarizing or repolarizing current expression. However, the physiological significance of this local variation remains unclear. It has been demonstrated that spatial AP duration variability is a significant contributor to the thoracic vein-derived arrhythmogenity [30]. It is possible to speculate that AP variations revealed in our experiments reflect a high electrophysiological heterogeneity of AZV tissue in particular and caval vein myocardium in general.
The resting membrane potential was depolarized in AZV if compared to the atrial myocardium under steady-state electrical pacing. We have also shown an additional spontaneous depolarization of RMP in AZV, which occurs in quiescent preparations after the pacing termination. The resting membrane potential in the quiescent AZV was significantly positive compared to RMP in a quiescent atrial myocardium. A similar RMP shift was reported previously in the pulmonary veins of different species including rats [5, 23, 25, 28]. The instability and reduced level of RMP was demonstrated in rabbit caval vein myocardium in several studies by the group of Ito and colleagues [31, 32]. The low density of the Kir2.X channels and inwardly rectifier I K1 [11], increased resting Na+ permeability [33] and enhanced chloride conduction [34] were suggested as the mechanisms for RMP depolarization in quiescent rat PV. The reasons for the RPM depolarization in quiescent AZV is unclear; however, it is possible to speculate that one or several of the mentioned mechanisms contribute to the described phenomenon. It is possible to hypothesize that an altered regulation, reduced density or turnover of Na/K-ATPase may also contribute to the dependence of RMP on pacing in the rat AZV. A termination of the Na+ entry through sodium channels upon quiescence onset could lead to the decrease of the Na/K-ATPase activity, which, in turn, could result in RMP depolarization, while resumption of the pacing causes pump stimulation and RMP hyperpolarization in the AZV. It should be noted that the resumption of the pacing in our experiments caused a shift of RMP to the more negative values. The mentioned mechanism is well known in latent pacemakers [35].
The AZV is the only remnant of the embryonic posterior cardinal veins in adult mammals. The cardinal veins drain the venous pole of the heart in the early stages of development in vertebrates [36, 37]. The myocardial sleeves of the cardinal veins and the embryonic venous pole of the heart share the same origin. Thus, the cardiac tissue in the AZV probably originates from the cells related to the precursors of the primary heart pacemaker–sinoatrial node (SAN). It is well known that the SAN is characterized by reduced Kir2.X/I K1 and Na/K-ATPase and low RMP. Undoubtedly, SAN and AZV myocardium in adult animals are very distinct electrophysiologically. However, the observed phenomenon allows the suggestion that the adult AZV myocardium partially retains the properties of the common embryonic precursor tissue. It has been recently established that a pacemaker tissue in SAN lacks transcription factor Nkx-2-5 and demonstrates a high level of Tbx3 and Tbx18 [38, 39]. It has also been shown that cardiac tissue in PV is characterized by the altered level of transcription factors such as Nkx-2-5, Tbx3 and Pitx2 in comparison with the atrial myocardium [39, 40]. In addition, it has been hypothesized that the transcriptional factors govern the electrophysiological phenotype of a cardiac tissue, and, in particular, that a reduced Nkx-2-5 protein level switches the PV myocardium to the pacemaker-resembling phenotype [41] and facilitates automaticity and conduction abnormalities in this tissue. Therefore, the embryonic origin allows the suggestion of a high tendency to automaticity and disturbed conduction of the cardiac tissue in AZV.
It was surprising that, despite reduced RMP and AP heterogeneity, the rat AZV demonstrated “quazy-normal” conduction of excitation without blocks or other abnormalities at least under steady-state pacing. The myocardial coating that completely surrounds the vein and is composed of several cardiomyocyte layers has been described in the rat AZV in previous investigations [16]. The lack of gaps inthe cardiac tissue and structural homogeneity probably underlie the atrial character of the conduction in the AZV. An observed pattern of the conduction also allows the assumption of atrial-like electrical coupling of the cardiomyocytes in AZV; however, this suggestion needs to be tested.
In addition, our experiments have demonstrated that the AZV myocardium is electrically coupled to the caval vein myocardial sleeve and to the atrial tissue: AZV excitation was observed when it was initiated by pacing from the atrial part of the preparations. It should be noted that AZV cardiac tissue lacks spontaneous AP under basal conditions. Therefore, we can speculate that AZV myocardium activation occurs in response to SAN-initiated excitation waves in vivo under basal conditions at least in the rat. However, the role of AZV myocardial sleeves in vivo both under basal conditions and sympathetic or adrenergic activation remains unclear. Nevertheless, several speculative hypotheses could be suggested. It has been demonstrated that the myocardial coating of the rat AZV of producing cardiac type twitch [17]. Therefore, it is possible that AZV myocardium act as a “valve” that prevents a back flow of blood during atrial contraction. Another potential role is that the contraction of the AZV in a combination with the caval vein increases atria blood filling under the high heart rate intrinsic to the rodents. The contribution of the AZV-caused atria filling could be increased under sympathetic activation or adrenergic stimulation which is accompanied by a growth of the heart rhythm. This suggestion could be supported by the previous observation that the contractility of the murine AZV increases in response to the adrenergic stimulation [17].
The adrenergic stimulation caused significant alteration of AZV electrical activity in our experiments. While noradrenaline causeda biphasic effect, that of hyperpolarization which is followed by depolarization and a spontaneous firing, selective α1-adrenoreceptor or β-adrenoreceptor agonist induced monophasic effects. In our study, phenylephrine caused only RMP depolarization, while isoproterenol only hyperpolarization. The opposite effects of NA on RMP are obviously mediated by the different adrenergic receptor activation. Noradrenaline failed to depolarize RMP in AZV in the presence of α1-antagonist prazosin in our experiments. A similar two-phase effect of NA on RMP has been observed previously in the rat pulmonary vein myocardium [42]. The dose-dependency of NE depolarizing/hyperpolarizing effects in the AZV probably results from the prevalence of the β-adrenoreceptors in the rat myocardium. Both β1 and β2-adrenoreceptors are abundantly presented in the heart at a ratio of 5:1, while α-adrenoreceptors only account for approximately 15% of cardiac adrenergic receptors [43, 44].
The depolarizing α1-adrenoreceptor-dependent effect of NE and PHE on RMP in quiescent AZV preparations may be associated with the inhibition of I K1 [45]. It should be noted that NA also affects chloride currents and probably modulates RMP via this mechanism [31]. In contrast, NA and ISO-induced hyperpolarization in AZV could result from a stimulation of Na–K-ATPase [46], background I KAch augmentation [47] or the suppression of a chloride conductance. In addition, the ISO-caused RMP shift could occur from calcium-dependent potassium currents (I K,Ca) activation, since it is well known that β-adrenoreceptor stimulation mobilizes intracellular calcium and I K,Ca have been identified in rodent and human heart [48, 49].
As has been mentioned above, NA-induced RMP alteration in AZV was followed by a spontaneous firing. An automatic activity in response to adrenergic stimulation has been demonstrated in previous investigations in canine [20], murine [28], rat [23, 42], guinea pig [26], and rabbit [27] PV, and also in canine superior CV myocardium [11]. We observed NA-induced automaticity in 7 out of 21 (33%) AZV preparations (Table 1). Thereby, automatic APs were initiated by NA stimulation in a minor part of our experiments. The adrenergic automaticity was observed in the predominant part of experiments with rat PV cardiac tissue [23]. It is possible to speculate that rat AZV is lesser prone to the automaticity than PV myocardium. However, similarly to the rat PV, the automatic activity in the rat AZV cardiac tissue manifested in the form of repetitive AP bursts of various lengths.
It should be noted that we observed only 1 case (out of 9) of firing initiation in AZV in response to the PHE application. Although some researchers have revealed the ability to induce spontaneous firing in different regions of the myocardium by β-agonist isoproterenol [12, 50, 51], we did not observe this effect in the rat AZV. Thus, a mechanism of adrenergic automaticity in AZV involves simultaneous α1- and β-adrenoreceptors activation and both types of the adrenergic receptors are curtailed for the firing initiation. It is also possible to hypothesize that a1-adrenoreceptor causes RMP depolarization while β-adrenoreceptor induces calcium mobilization, which is necessary for a firing triggering. It has been demonstrated in a number of investigations that calcium mobilization by noradrenaline underlies both the regulation of a normal SAN pacemaking and arrhythmogenic automaticity in various cardiac regions [52]. A Na+–Ca2+ exchanger could play a key role in the adrenergic AP triggering in the rat AZV, as has been demonstrated in the rat pulmonary veins [53].
In addition, it seems that the pattern of the firing is highly dependent on the delicate balance of the α1- and β-adrenoreceptor activation and stimulation of the corresponding intracellular regulatory pathways in AZV myocardium. In our experiments, both α- and β-adrenoreceptor antagonists were effective in the termination of the firing in the rat AZV. Either propranolol or prazosin application led to the termination of the NA-induced automatic AP bursts. It has been reported that antagonism of either α- or β-adrenoreceptors abolished NE-caused automatic firing in the guinea pig and rat pulmonary veins, another region of the thoracic venous vessels [42, 53, 54]. Nevertheless, the dual blockade of both types of adrenoreceptors could be most effective in the suppression of thoracic vein-derived automaticity and arrhythmogenic RMP changes. Our study has also demonstrated that adrenergic blockade is effective not only in the pulmonary vein myocardium but also in caval vein derivatives.
In conclusion, the atrial-like AP and conduction of the excitation were observed at least in a part of the rat AZV under steady-state electrical pacing, and thus rat AZV partially resembles atrial myocardium. However, termination of the pacing resulted in spontaneous RMP depolarization in the AZV myocardium similar to the rat pulmonary vein. No spontaneous AP were recorded in quiescent AZV preparations under basal conditions; nevertheless, automaticity in the form of AP bursts was induced by adrenergic stimulation in part of the preparations. NA-induced automaticity in AZV depends on simultaneous α1- and β-adrenoreceptor activation. It is possible to speculate that a relationship to a tissue of embryonic cardinal veins and sinus venosus could underlie bioelectrical properties of the AZV myocardium.
References
Nathan H, Eliakim M (1966) The junction between the left atriuni and the pulmonary veins an anatomic study of human hearts. Circulation 34:412–422. doi:10.1161/01.CIR.34.3.412
Zipes DP, Knope RF (1972) Electrical properties of the thoracic veins. Am J Cardiol 29:372–376. doi:10.1016/0002-9149(72)90533-4
Marshall J (1850) On the development of the great anterior veins in man and mammalia; including an account of certain remnants of foetal structure found in the adult, a comparative view of these great veins in the different mammalia, and an analysis of their occasional peculiarities in the human subject. Philos Trans R Soc Lond 140:133–170
Spach MS, Barr RC, Jewett PH (1972) Spread of excitation from the atrium into thoracic veins in human beings and dogs. Am J Cardiol 30:844–854. doi:10.1016/0002-9149(72)90009-4
Cheung DW (1980) Electrical activity of the pulmonary vein and its interaction with the right atrium in the guinea-pig. J Physiol 314:445–456
Ito M, Arita M, Saeki K, Tanoue M (1967) Functional properties of sinocaval conduction. Jpn J Physiol 17:174–189
Brunton T, Fayrer J (1876) Note on independent pulsation of the pulmonary veins and vena cava. Proc R Soc Lond 25:174–176. doi:10.1098/rspl.1876.0041
Haissaguerre M, Jais P, Shah DC et al (1998) Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339:659–666
Cheung DW (1981) Pulmonary vein as an ectopic focus in digitalis-induced arrhythmia. Nature 294:582–584
Arita M, Saeki K, Tanoue M et al (1966) Studies on transmembrane action potentials and mechanical responses of the venae cavae abd atria of the rabbit. Jpn J Physiol 16:462–480. doi:10.2170/jjphysiol.16.462
Chen YJ, Chen YC, Yeh HI et al (2002) Electrophysiology and arrhythmogenic activity of single cardiomyocytes from canine superior vena cava. Circulation 105:2679–2685. doi:10.1161/01.CIR.0000016822.96362.26
Kurotobi T, Kino N, Tonomura D, Shimada Y (2015) Macro-reentrant atrial tachycardia conducting through a left superior vena cava after catheter ablation in a patient with paroxysmal atrial fibrillation. Can J Cardiol 31(103):e13–e15. doi:10.1016/j.cjca.2014.09.024
Tsai C-F, Tai C-T, Hsieh M-H et al (2000) Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava electrophysiological characteristics and results of radiofrequency ablation. Circulation 102:67–74. doi:10.1161/01.CIR.102.1.67
Kracklauer MP, Feng HZ, Jiang W et al (2013) Discontinuous thoracic venous cardiomyocytes and heart exhibit synchronized developmental switch of troponin isoforms. FEBS J 280:880–891. doi:10.1111/febs.12076
Hashizume H, Ushiki T, Abe K (1995) A histological study of the cardiac muscle of the human superior and inferior vena cavae. Arch Histol Cytol 58:457–464
Cullinan V, Campbell JH, Mosse PR, Campbell GR (1986) The morphology and cell culture of the striated musculature of the rat azygos vein. Cell Tissue Res 243:185–191
Liu R, Feng H-Z, Jin J-P (2014) Physiological contractility of cardiomyocytes in the wall of mouse and rat azygos vein. Am J Physiol Cell Physiol 306:C697–C704. doi:10.1152/ajpcell.00004.2014
Armour J, Murphy D, Yuan B et al (1997) Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec 247:289–298. doi:10.1002/(SICI)1097-0185(199702)247:2<289:AID-AR15>3.0.CO;2-L
Tan AY, Li H, Wachsmann-Hogiu S et al (2006) Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction. implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol 48:132–143. doi:10.1016/j.jacc.2006.02.054
Patterson E, Po SS, Scherlag BJ, Lazzara R (2005) Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Hear Rhythm 2:624–631. doi:10.1016/j.hrthm.2005.02.012
Sharifov OF, Fedorov VV, Beloshapko GG et al (2004) Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol 43:483–490. doi:10.1016/j.jacc.2003.09.030
Lu Z, Scherlag BJ, Lin J et al (2009) Autonomic mechanism for initiation of rapid firing from atria and pulmonary veins: evidence by ablation of ganglionated plexi. Cardiovasc Res 84:245–252. doi:10.1093/cvr/cvp194
Egorov YV, Kuz’min VS, Glukhov AV, Rosenshtraukh LV (2015) Electrophysiological characteristics, rhythm, disturbances and conduction discontinuities under autonomic stimulation in the rat pulmonary vein myocardium. J Cardiovasc Electrophysiol 26:1130–1139. doi:10.1111/jce.12738
Sicouri S, Blazek J, Belardinelli L, Antzelevitch C (2012) Electrophysiological characteristics of canine superior vena cava sleeve preparations. Effect of ranolazine. Circ Arrhythmia Electrophysiol 5:371–379. doi:10.1161/CIRCEP.111.969493
Ehrlich JR, Cha T-J, Zhang L et al (2003) Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J Physiol 551:801–813. doi:10.1111/j.1469-7793.2003.00801.x
Namekata I, Tsuneoka Y, Takahara A et al (2009) Involvement of the Na(+)/Ca(2+) exchanger in the automaticity of guinea-pig pulmonary vein myocardium as revealed by SEA0400. J Pharmacol Sci 110:111–116. doi:10.1254/jphs.08159SC
Chen YJ, Chen SA, Chen YC et al (2002) Electrophysiology of single cardiomyocytes isolated from rabbit pulmonary veins: implication in initiation of focal atrial fibrillation. Basic Res Cardiol 97:26–34. doi:10.1007/s395-002-8384-6
Tsuneoka Y, Kobayashi Y, Honda Y et al (2012) Electrical activity of the mouse pulmonary vein myocardium. J Pharmacol Sci 119:287–292. doi:10.1254/jphs.12062SC
Miyauchi Y, Hayashi H, Miyauchi M et al (2005) Heterogeneous pulmonary vein myocardial cell repolarization implications for reentry and triggered activity. Heart Rhythm 2:1339–1345. doi:10.1016/j.hrthm.2005.09.015
Kim B-S, Kim Y-H, Hwang G-S et al (2002) Action potential duration restitution kinetics in human atrial fibrillation. J Am Coll Cardiol 39:1329–1336. doi:10.1016/S0735-1097(02)01760-6
Yanaga T, Ito M, Saeki K et al (1966) The ectopic pacemaker formation in the left superior vena cava proximal to the heart and the genesis of cardiac arrhythmias. Jpn Heart J 7:505–511
Ito M, Arita M, Saeki K, Tanoue M, Fukushima I (1967) Functional properties of sinocaval conduction. Jpn J Physiol 17(2):174–189
Malécot CO, Bredeloux P, Findlay I, Maupoil V (2015) A TTX-sensitive resting Na+ permeability contributes to the catecholaminergic automatic activity in rat pulmonary vein. J Cardiovasc Electrophysiol 26:311–319. doi:10.1111/jce.12572
Okamoto Y, Kawamura K, Nakamura Y, Ono K (2014) Pathological impact of hyperpolarization-activated chloride current peculiar to rat pulmonary vein cardiomyocytes. J Mol Cell Cardiol 66:53–62. doi:10.1016/j.yjmcc.2013.11.002
Antzelevitch C, Burashnikov A (2011) Overview of basic mechanisms of cardiac arrhythmia. Card Electrophysiol Clin 3(1):23–45
Hikspoors JPJM, Mekonen HK, Mommen GMC et al (2016) Infrahepatic inferior caval and azygos vein formation in mammals with different degrees of mesonephric development. J Anat 228:495–510. doi:10.1111/joa.12423
Butler H (1950) The development of the azygos veins in the albino rat. J Anat 84(2):83–94
Moorman AFM, Anderson RH (2010) Development of the pulmonary vein. Int J Cardiol 147:182. doi:10.1016/j.ijcard.2010.12.034
Mommersteeg MTM, Brown NA, Prall OWJ et al (2007) Pitx2c and Nkx2-5 are required for the formation and identity of the pulmonary myocardium. Circ Res 101:902–909. doi:10.1161/CIRCRESAHA.107.161182
Mommersteeg MTM, Christoffels VM, Anderson RH, Moorman AFM (2009) Atrial fibrillation: a developmental point of view. Heart Rhythm 6:1818–1824. doi:10.1016/j.hrthm.2009.07.011
Postma AV, Dekker LRC, Soufan AT, Moorman AFM (2009) Developmental and genetic aspects of atrial fibrillation. Trends Cardiovasc Med 19:123–130. doi:10.1016/j.tcm.2009.07.003
Doisne N, Maupoil V, Cosnay P, Findlay I (2009) Catecholaminergic automatic activity in the rat pulmonary vein: electrophysiological differences between cardiac muscle in the left atrium and pulmonary vein. Am J Physiol Heart Circ Physiol 297:H102–H108. doi:10.1152/ajpheart.00256.2009
Bengel FM, Schwaiger M (2004) Assessment of cardiac sympathetic neuronal function using PET imaging. J Nucl Cardiol 11:603–616. doi:10.1016/j.nuclcard.2004.06.133
Bristow MR (1993) Changes in myocardial and vascular receptors in heart failure. J Am Coll Cardiol 22:A61–A71. doi:10.1016/0735-1097(93)90465-D
Wang H, Yang B, Zhang Y et al (2001) Different subtypes of α1 -adrenoceptor modulate different K+ currents via different signaling pathways in canine ventricular myocytes. J Biol Chem 276:40811–40816. doi:10.1074/jbc.M105572200
Diacono J, Ditrich J, Lajoix H (1986) Opposite effects of adrenaline and ouabain on the resting potential of rat atrial cells. Life Sci 39:2541–2550
Yeh YH, Ehrlich JR, Qi X et al (2007) Adrenergic control of a constitutively active acetylcholine-regulated potassium current in canine atrial cardiomyocytes. Cardiovasc Res 74:406–415. doi:10.1016/j.cardiores.2007.01.020
Xu Y, Tuteja D, Zhang Z et al (2003) Molecular identification and functional roles of a Ca(2+)-activated K+ channel in human and mouse hearts. J Biol Chem 278:49085–49094. doi:10.1074/jbc.M307508200
Skibsbye L, Poulet C, Diness JG et al (2014) Small-conductance calcium-activated potassium (SK) channels contribute to action potential repolarization in human atria. Cardiovasc Res 103:156–167. doi:10.1093/cvr/cvu121
Naggar I, Uchida S, Kamran H et al (2012) Autonomic boundary conditions for ventricular fibrillation and their implications for a novel defibrillation technique. J Physiol Sci 62:479–492. doi:10.1007/s12576-012-0225-8
Chen YJ, Chen SA, Chen YC et al (2001) Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: implication in initiation of atrial fibrillation. Circulation 104:2849–2854. doi:10.1161/hc4801.099736
Lakatta EG, Maltsev VA, Bogdanov KY et al (2003) Cyclic variation of intracellular calcium: a critical factor for cardiac pacemaker cell dominance. Circ Res 92:e45–e50. doi:10.1161/01.RES.0000055920.64384.FB
Okamoto Y, Takano M, Ohba T, Ono K (2012) Arrhythmogenic coupling between the Na+–Ca2+ exchanger and inositol 1,4,5-triphosphate receptor in rat pulmonary vein cardiomyocytes. J Mol Cell Cardiol 52(5):988–997. doi:10.1016/j.yjmcc.2012.01.007
Irie M, Tsuneoka Y, Shimobayashi M, Hasegawa N et al (2017) Involvement of alpha- and beta-adrenoceptors in the automaticity of the isolated guinea pig pulmonary vein myocardium. J Pharmacol Sci 133:247–253. doi:10.1016/j.jphs.2017.03.003
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study is supported by RFBR Grant 17-04-01921.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All experimental procedures were carried out in accordance with the Guide for the Care and the Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 2010) and approved by the Ethics Committee of the MSU Biological department.
About this article
Cite this article
Ivanova, A.D., Kuzmin, V.S. Electrophysiological characteristics of the rat azygos vein under electrical pacing and adrenergic stimulation. J Physiol Sci 68, 617–628 (2018). https://doi.org/10.1007/s12576-017-0569-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-017-0569-1