Abstract
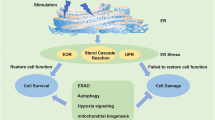
The endoplasmic reticulum (ER) is an intracellular organelle involved in biosynthesis and the secretory pathway. This organelle has many resident proteins including biosynthetic enzymes and secretory proteins. Recent studies have suggested that dysfunction of the ER or secretory pathway is involved in the pathogenesis of various human diseases. Some stresses acting on the ER, which are designated ER stress, induce the accumulation of unfolded/misfolded proteins in the ER, leading to cell death. Misfolded proteins are retained until they form their native conformation or returned to the cytosol for degradation by the proteasome. Among the ER-resident proteins, molecular chaperones prevent aggregation of proteins within the ER, and orchestrate the ER quality control systems. We have reported the roles of novel stress proteins, namely 150-kDa oxygen-regulated protein, 94-kDa glucose-regulated protein and RA410. These proteins are induced significantly by hypoxia or oxidative stress and have cytoprotective effects under these conditions. These findings suggest that hypoxia and oxidative stress target the ER and secretory pathway, resulting in ER stress, and that these proteins exert cytoprotective effects in various diseases associated with ER stress.








Similar content being viewed by others
References
Aleshin AN, Sawa Y, Kitagawa-Sakakida S et al (2005) 150-kDa oxygen-regulated protein attenuates myocardial ischemia-reperfusion injury in rat heart. J Mol Cell Cardiol 38(3):517–525
Bando Y, Ogawa S, Yamauchi A et al (2000) 150-kDa oxygen-regulated protein (ORP150) functions as a novel molecular chaperone in MDCK cells. Am J Physiol Cell Physiol 278(6):C1172–C1182
Bando Y, Katayama T, Kasai K, Taniguchi M, Tamatani M, Tohyama M (2003) GRP94 (94 kDa glucose-regulated protein) suppresses ischemic neuronal cell death against ischemia/reperfusion injury. Eur J Neurosci 18(4):829–840
Bando Y, Katayama T, Aleshin AN, Manabe T, Tohyama M (2004a) GRP94 reduces cell death in SH-SY5Y cells perturbated calcium homeostasis. Apoptosis 9(4):501–508
Bando Y, Tsukamoto Y, Katayama T et al (2004b) ORP150/HSP12A protects renal tubular epithelium from ischemia-induced cell death. FASEB J 18(12):1401–1403
Bando Y, Katayama T, Taniguchi M et al (2005a) RA410/Sly1 suppresses MPP+ and 6-hydroxydopamine-induced cell death in SH-SY5Y cells. Neurobiol Dis 18(1):143–151
Bando Y, Onuki R, Katayama T et al (2005b) Double-strand RNA dependent protein kinase (PKR) is involved in the extrastriatal degeneration in Parkinson’s disease and Huntington’s disease. Neurochem Int 46(1):11–18
Eletto D, Dersh D, Argon Y (2010) GRP94 in ER quality control and stress responses. Semin Cell Dev Biol 21(5):479–485
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397(6716):271–274
Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5(5):897–904
Haze K, Yoshida H, Yanagi H, Yura T, Mori K (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10(11):3787–3799
Honma Y, Kanazawa K, Mori T et al (1999) Identification of a novel gene, OASIS, which encodes for a putative CREB/ATF family transcription factor in the long-term cultured astrocytes and gliotic tissue. Brain Res Mol Brain Res 69(1):93–103
Hoseki J, Ushioda R, Nagata K (2010) Mechanism and components of endoplasmic reticulum-associated degradation. J Biochem 147(1):19–25
Hosokawa N, Wada I, Hasegawa K et al (2001) A novel ER alpha-mannosidase-like protein accelerates ER-associated degradation. EMBO Rep 2(5):415–422
Imaizumi K, Katayama T, Tohyama M (2001a) Presenilin and the UPR. Nat Cell Biol 3(5):E104
Imaizumi K, Miyoshi K, Katayama T et al (2001b) The unfolded protein response and Alzheimer’s disease. Biochim Biophys Acta 1536(2–3):85–96
Katayama T, Imaizumi K, Manabe T, Hitomi J, Kudo T, Tohyama M (2004) Induction of neuronal death by ER stress in Alzheimer’s disease. J Chem Neuroanat 28(1–2):67–78
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Investig 110:1389–1398
Kitao Y, Ozawa K, Miyazaki M et al (2001) Expression of the endoplasmic reticulum molecular chaperone (ORP150) rescues hippocampal neurons from glutamate toxicity. J Clin Invest 108(10):1439–1450
Kondo S, Murakami T, Tatsumi K et al (2005) OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat Cell Biol 7(2):186–194
Kuwabara K, Matsumoto M, Ikeda J et al (1996) Purification and characterization of a novel stress protein, the 150-kDa oxygen-regulated protein (ORP150), from cultured rat astrocytes and its expression in ischemic mouse brain. J Biol Chem 271(9):5025–5032
Machamer CE (2003) Golgi disassembly in apoptosis: cause or effect? Trends Cell Biol 13:279–281
Mancini M, Machamer CE, Roy S et al (2000) Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J Cell Biol 149(3):603–612
Matsuo N, Ogawa S, Takagi T et al (1997) Cloning of a putative vesicle transport-related protein, RA410, from cultured rat astrocytes and its expression in ischemic rat brain. J Biol Chem 272(26):16438–16444
Miyazaki M, Ozawa K, Hori O et al (2002) Expression of 150-kd oxygen-regulated protein in the hippocampus suppresses delayed neuronal cell death. J Cereb Blood Flow Metab 22(8):979–987
Murakami T, Saito A, Hino S et al (2009) Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol 11(10):1205–1211
Nakatani Y, Kaneto H, Kawamori D et al (2005) Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem 280(1):847–851
Nakatani Y, Kaneto H, Hatazaki M et al (2006) Increased stress protein ORP150 autoantibody production in Type 1 diabetic patients. Diabet Med 23(2):216–219
Onuki R, Bando Y, Suyama E et al (2004) An RNA-dependent protein kinase is involved in tunicamycin-induced apoptosis and Alzheimer’s disease. EMBO J 23(4):959–968
Ozawa K, Kuwabara K, Tamatani M et al (1999) 150-kDa oxygen-regulated protein (ORP150) suppresses hypoxia-induced apoptotic cell death. J Biol Chem 274(10):6397–6404
Ozawa K, Tsukamoto Y, Hori O et al (2001a) Regulation of tumor angiogenesis by oxygen-regulated protein 150, an inducible endoplasmic reticulum chaperone. Cancer Res 61(10):4206–4213
Ozawa K, Kondo T, Hori O et al (2001b) Expression of the oxygen-regulated protein ORP150 accelerates wound healing by modulating intracellular VEGF transport. J Clin Invest 108(1):41–50
Ozawa K, Miyazaki M, Matsuhisa M et al (2005) The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes 54(3):657–663
Ron D (2002) Translational control in the endoplasmic reticulum stress response. J Clin Invest 110(10):1383–1388
Sanson M, Augé N, Vindis C et al (2009) Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: prevention by oxygen-regulated protein 150 expression. Circ Res 104(3):328–336
Tamatani M, Matsuyama T, Yamaguchi A et al (2001) ORP150 protects against hypoxia/ischemia-induced neuronal death. Nat Med 7(3):317–323
Tsukamoto Y, Kuwabara K, Hirota S et al (1996) 150-kD oxygen-regulated protein is expressed in human atherosclerotic plaques and allows mononuclear phagocytes to withstand cellular stress on exposure to hypoxia and modified low density lipoprotein. J Clin Invest 98(8):1930–1941
Tsukamoto Y, Kuwabara K, Hirota S et al (1998) Expression of the 150-kd oxygen-regulated protein in human breast cancer. Lab Invest 78(6):699–706
Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D (1998) Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J 17(19):5708–5717
Yoshida H, Haze K, Yanagi H, Yura T, Mori K (1998) Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem 273(50):33741–33749
Acknowledgments
I greatly thank Prof. Masaya Tohyama (Osaka University Graduate School of Medicine) and Dr. Satoshi Ogawa for their advice and valuable discussions. I also thank Profs. Shigetaka Yoshida (Asahikawa Medical University) and Taiichi Katayama (United Graduate School of Child Development, Osaka University, Kanazawa University and Hamamatsu University Graduate School of Medicine) for their helpful discussions. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest
The author reports no financial or any other conflicts of interest with the contents of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bando, Y. The functional role of stress proteins in ER stress mediated cell death. Anat Sci Int 87, 14–23 (2012). https://doi.org/10.1007/s12565-011-0127-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-011-0127-5