Abstract
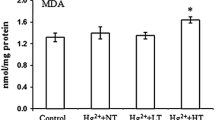
Recent studies on the effects of temperature on the antioxidant system of fish have been conducted mostly in vivo. In vivo experimental results are influenced by many factors, and can vary widely. Hence, experiments at the cellular level can provide a new direction for scientific research. The purpose of this study was to investigate the mechanism of the antioxidant system in response to temperature changes in grass carp Ctenopharyngodon idellus kidney (CIK) cells. CIK cells were exposed to culture temperatures of 20 °C, 24 °C, 28 °C, 32 °C, and 36 °C for 24 h, and the results showed that heat stress significantly increased the level of reactive oxygen species (ROS), which further led to increased content of malondialdehyde (MDA) and protein carbonyl. The increase of the ratio of Rh123 fluorescence indicated a decrease in mitochondrial membrane potential (\(\Delta \Psi {\text{m}}\)), demonstrating that the changes in temperature destroyed the mitochondrial membrane of CIK cells. The acute temperature stress increased the activity of superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and glutathione reductase (GR), and improved total antioxidant capacity (TAC) and glutathione (GSH). The relative mRNA expression levels of Cu–Zn sod, gpx, and cat were significantly increased with the variation in temperature. In conclusion, the changes in temperature disturbed the homeostasis of the CIK cells, destroyed the mitochondrial membrane, and enhanced the activity of major antioxidant enzymes to resist oxidative stress.





Similar content being viewed by others
Data availability
There are no linked research data sets for this submission. The data will be made available on request.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
An MI, Choi CY (2010) Activity of antioxidant enzymes and physiological responses in ark shell Scapharca broughtonii exposed to thermal and osmotic stress: effects on hemolymph and biochemical parameters. Comp Biochem Phys B 155:34–42
Babich H, Palace MR, Stern A (1993) Oxidative stress in fish cells. In vitro studies. Arch Environ Con Tox 24:173–178
Bagnyukova TV, Lushchaka OV, Storey KB, Lushchak VI (2007) Oxidative stress and antioxidant defense responses by goldfish tissues to acute change of temperature from 3 to 23 °C. J Therm Biol 32:227–234
Benzie IF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27
Beyer WF Jr, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Cheng CH, Yang FF, Liao SA, Miao YT, Ye CX, Wang AL, Tan JW, Chen XY (2015) High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J Therm Biol 53:172–179
Coelho ÉMP, Barbosa MC, Mito MS, Mantovanelli GC, Oliveira RS Jr, Ishii-Iwamoto EL (2017) The activity of the antioxidant defense system of the weed species Senna obtusifolia L. and its resistance to allelochemical stress. J Chem Ecol 43:725–738
Cui YT, Liu B, Xie J, Xu P, Tsion HM, Zhang YY (2013) The effect of hyperthermia on cell viability, oxidative damage, and heat shock protein expression in hepatic cells of grass carp Ctenopharyngodon idellus. J Therm Biol 38:355–361
Dadras H, Dzyuba V, Cosson J, Golpour A, Dzyuba B (2016) The in vitro effect of temperature on motility and antioxidant response of common carp Cyprinus carpio spermatozoa. J Therm Biol 59:64–68
Drotar A, Phelps P, Fall R (1985) Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci 42:35–40
Feidantsis K, Poertner HO, Lazou A, Kostoglou B, Michaelidis B (2009) Metabolic and molecular stress responses of the gilthead seabream sparus aurata during long-term exposure to increasing temperatures. Mar Biol 156(4):797–809
Feng GP, Zhuang P, Zhang LZ, Duan M, Liu JY (2012) Effects of temperature on oxidative stress biomarkers in juvenile Chinese sturgeon Acipenser sinensis under laboratory conditions. Adv Mater Res 343–344:497–504
Garcia SF, de Lima BC, Tie OE, Romagueira BL, Lúcia KA, Tadeu RF (2008) Antioxidant defenses and biochemical changes in pacu Piaractus mesopotamicus in response to single and combined copper and hypoxia exposure. Comp Biochem Physiol C Toxicol Pharmacol 147(1):43–51
Guo H, Lin W, Hou J, Wang L, Zhang D, Wu X, Li L, Li D (2018) The Protective Roles of Dietary Selenium Yeast and Tea Polyphenols on Growth Performance and Ammonia Tolerance of Juvenile Wuchang Bream Megalobrama amblycephala. Front Physiol 9:1371
Halestrap AP (2010) A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans 38:841–860
Hansen BH, Rømma S, Garmo ØA, Olsvik PA, Andersen RA (2006) Antioxidative stress proteins and their gene expression in brown trout Salmo trutta from three rivers with different heavy metal levels. Comp Biochem Phys C 143:263–274
Kailasam M, Kaneko G, Oo AKS, Ozaki Y, Thirunavukkarasu AR, Watabe S (2011) Effects of calorie restriction on the expression of manganese superoxide dismutase and catalase under oxidative stress conditions in the rotifer Brachionus plicatilis. Fish Sci 77:403–409
Kammer AR, Orczewska JI, O’Brien KM (2011) Oxidative stress is transient and tissue specific during cold acclimation of three-spine stickleback. J Exp Biol 214:1248–1256
Klein RD, Borges VD, Rosa CE, Colares EP, Robaldo RB, Martinez PE, Bianchini A (2017) Effects of increasing temperature on antioxidant defense system and oxidative stress parameters in the Antarctic fish Notothenia coriiceps and Notothenia rossii. J Therm Biol 68:110–118
Kültz D (2005) Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 67:225–257
Lankford SE, Adams TE, Cech JJ Jr (2003) Time of day and water temperature modify the physiological stress response in green sturgeon Acipenser medirostris. Comp Biochem Phys A 135:291–302
Leggatt RA, Brauner CJ, Schulte PM, Iwama GK (2007) Effects of acclimation and incubation temperature on the glutathione antioxidant system in killifish and RTH-149 cells. Comp Biochem Phys A 146:317–326
Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68:253–278
Li ZH, Zlabek V, Grabic R, Li P, Randak T (2010) Modulation of glutathione-related antioxidant defense system of fish chronically treated by the fungicide propiconazole. Comp Biochem Physiol C 152:392–398
Lin W, Hou J, Guo H, Li L, Wang L, Zhang D, Li D, Tang R (2018) The synergistic effects of waterborne microcystin-LR and nitrite on hepatic pathological damage, lipid peroxidation and antioxidant responses of male zebrafish. Environ Pollut 235:197–206
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Luo W, Xu Y, Liu XJ, Wang CF (2017) Effect of water temperature on serum content of reactive oxygen species and antioxidant defense system in grass carp Ctenopharyngodon idella. Freshw Fish 47(04):3–7
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Lushchak VI, Bagnyukova TV (2006) Temperature increase results in oxidative stress in goldfish tissues. 1. Indices of oxidative stress. Comp Biochem Phys C 143:30–35
Lushchak VI, Bagnyukova TV, Husak VV, Luzhna LI, Lushchak OV, Storey KB (2005) Hyperoxia results in transient oxidative stress and an adaptive response by antioxidant enzymes in goldfish tissues. Int J of Biochem Cell B 37:1670–1680
Malek RL, Sajadi H, Abraham J, Grundy MA, Gerhard GS (2004) The effects of temperature reduction on gene expression and oxidative stress in skeletal muscle from adult zebrafish. Comp Biochem Phys C 138:363–373
Marchant RJ, Al-Fageeh MB, Underhill MF, Racher AJ, Smales CM (2008) Metabolic rates, growth phase, and mRNA levels influence cell-specific antibody production levels from in vitro-cultured mammalian cells at sub-physiological temperatures. Mol Biotechnol 39:69–77
Murphy BJ, Sato BG, Dalton TP, Laderoute KR (2005) The metal-responsive transcription factor-1 contributes to HIF-1 activation during hypoxic stress. Biochem Bioph Res Co 337:860–867
Niyogi S, Biswas S, Sarker S, Datta AG (2001) Seasonal variation of antioxidant and biotransformation enzymes in barnacle Balanus balanoides and their relation with polyaromatic hydrocarbons. Mar Environ Res 52:13–26
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Parihar MS, Dubey AK (1995) Lipid peroxidation and ascorbic acid status in respiratory organs of male and female freshwater catfish Heteropneustes fossilis exposed to temperature increase. Comp Biochem Phys C 112:309–313
Prokopenko VM, Partsalis GK, Pavlova NG, Burmistrov SO, Arutyunyan AV (2002) Glutathione-dependent system of antioxidant defense in the placenta in preterm delivery. Bull Exp Biol Med 133:442–443
Qiang J, Zhong CY, Bao JW, Liang M, Liang C, Li HX, He J, Xu P (2019) The effects of temperature and dissolved oxygen on the growth, survival and oxidative capacity of newly hatched hybrid yellow catfish larvae Tachysurus fulvidraco♀ ×Pseudobagrus vachellii♂. J Therm Biol 86:102436
Rocha-Santos C, Bastos FF, Dantas RF, Hauser-Davis RA, Rodrigues LC, Bastos VLFC, Bastos JC (2018) Glutathione peroxidase and glutathione S-transferase in blood and liver from a hypoxia tolerant fish under oxygen deprivation. Ecotoxicol Environ Saf 163:604–611
Rossati A (2017) Global Warming and Its Health Impact. Int J Occup Environ Med 8:7–20
Scaion D, Vettier A, Sébert P (2008) Pressure and temperature interactions on aerobic metabolism in migrating silver eels: results in vitro. Undersea Hyperbar M 35:27–32
Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, Dmitriev AA (2019) ROS generation and antioxidant defense systems in normal and malignant cells. Oxid Med Cell Longev 2019:6175804
Song ZM, Liu JY, Zhuang P, Wang Y, Zhang LZ, Hu Y, Gong P (2015) Influence of low-temperature stress on the antioxidant enzymes activities and malondialdehyde contents in liver of juvenile Siganus guttatas. Mar Fish 37(02):142–150
Tanaka R, Hatate H, Ito M, Nakamura T (2006) Elevation of lipid peroxide level and production of hydroxy lipids in cultured Hepa-T1 cells by oxidative stressors. Fish Sci 72:665–672
Tseng YC, Chen RD, Lucassen M, Schmidt MM, Dringen R, Abele D, Hwang PP (2011) Exploring uncoupling proteins and antioxidant mechanisms under acute cold exposure in brains of fish. PLoS ONE 6:e18180
Van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Phar 13:57–149
Wilhelm Filho D (1996) Fish antioxidant defenses–a comparative approach. Braz J Med Biol Res 29:1735–1742
Xu QQ, Han BS, Luo JT, Yan L, Hou YW, Hu P, Zhang JF (2016) Effects of cold stress on ROS production and expression of MAPK proteins in zebrafish ZF4 cells. J Fish Sci China 23(04):771–776
Yang S, Yan T, Zhao LL, Wu H, Du ZJ, Yan TM, Xiao Q (2018) Effects of temperature on activities of antioxidant enzymes and Na+/K+-ATPase, and hormone levels in Schizothorax prenanti. J Therm Biol 72:155–160
Acknowledgements
This research was supported by the National Natural Science Foundation (no. 31502140) and the China Agriculture Research System of MOF and MARA (CARS-45-24).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, P., Yin, X., Li, D. et al. Acute temperature changes induce an oxidative stress response in kidney cells of grass carp Ctenopharyngodon idellus. Fish Sci 87, 775–784 (2021). https://doi.org/10.1007/s12562-021-01545-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-021-01545-2