Abstract
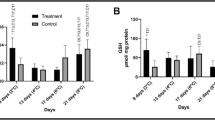
Temperature shock is a major natural cause of mass mortality of global fish. Nile tilapia Oreochromis niloticus, one of the most widely cultured species, with high resistance against severe environmental conditions, is also affected by rapid temperature changes. This research was conducted to investigate histopathological changes and oxidative stress in O. niloticus in response to temperature shock. The malondialdehyde levels in the serum, gills and brain, the histopathology of the gills and brain, and the histochemical characterization of the gills’ mucosubstances were measured in the fish exposed to heat shock and cold shock conditions. The results showed that the fish could not survive under 37 °C for 24 hours. Malondialdehyde levels in the gills, brain and serum increased significantly when compared to those of the controls. Moreover, histopathological changes and a decrease in the number of neutral and acidic mucous cells was observed in the gills of fish in both the heat shock and cold shock groups. Histopathological alteration, vacuolated neuropil in the brain, was observed only in the fish in the cold shock groups. The results from this study indicate that rapid 4 °C changes in water temperature (25–21 and 25–29 °C) evoked oxidative stress and histological damage to O. niloticus, whereas extreme 12 °C changes (25–13 and 25–37 °C) severely affected their oxidative stress and histopathological condition.






Similar content being viewed by others
References
Aboka ER, Jian Z, Shengming S, Wuxiao Z, Han Y, Li SY, Jiahui Y, Jiabin Z (2017) Histopathological changes in gills, liver, and kidney tissues of bighead carp (Aristichthys nobilis) due to the effects of acute high-temperature stress. Isr J Aquacult-Bamid 69:2–10
Ahmed RG (2005) Is there a balance between oxidative stress and antioxidant defense system during development? Med J Islamic Acad Sci 15:55–63
Al-Zaidy KJ (2013) First record of Tilapia zilli (Gewais, 1848) in Al-delmj marsh west Al-Diwania city middle of Iraq. DASJ 5:9–16
Arscott DB, Tockner K, Ward JV (2001) Thermal heterogeneity along a braided floodplain river (Tagliamento River, northeastern Italy). Can J Fish Aquat Sci 58:2359–2373
Avella M, Berhaut J, Bornancin M (1993) Salinity tolerance of two tropical fishes Oreochromis aureus and O. niloticus. I. Biochemical and morphological changes in the gill epithelium. J Fish Biol 42:243–254
Azaza MS, Dhraïef MN, Kraïem MM (2008) Effects of water temperature on growth and sex ratio of juvenile Nile tilapia Oreochromis niloticus (Linnaeus) reared in geothermal waters in southern Tunisia. J Therm Biol 33:98–105
Bagnyukova TV, Lushchak OV, Storey KB, Lushchak VI (2007) Oxidative stress and antioxidant defense responses by goldfish tissues to acute change of temperature from 3 to 23 °C. J Therm Biol 32:227–234
Baleta FN, Bolaños JM, Medrano WC (2019) Assessment of tilapia cage farming practices in relation to the occurrence of fish mortality along the fish cage belt at Magat Reservoir, Philippines. J Fish Environ 43:1–13
Bansil R, Turner BR (2005) Mucin structure, aggregation, physiological functions and biomedical applications. Curr Opin Colloid In 11:164–170
Beitinger TL, Bennett WA, McCauley RW (2000) Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fish 58:237–275
Berntssen MHG, Kroglund F, Rosseland BO, Wendelaar Bonga SE (1997) Responses of skin mucous cells to aluminum exposure at low pH in Atlantic salmon (Salmo salar) smolts. Can J Fish Aquat Sci 54:1039–1045
Bishai HM (1965) Resistance of Tilapia nilotica L. to high temperatures. Hydrobiologia 25:473–488
Boveris A (1977) Mitochondrial production of superoxide radical and hydrogen peroxide. Adv Exp Med Biol 78:67–82
Boyd CE, Tucker CS (1998) Pond aquaculture water quality management. Springer, New York
Boyd RB, De Vries AL, Eastman JT, Pietra GG (1980) The secondary lamellae of the gills of cold water (high latitude) teleost. A comparative light and electron microscopic study. Cell Tissue Res 213:361–510
Cerqueira CCC, Fernandes MN (2002) Gill tissue recovery after copper exposure and blood parameter responses in the tropical fish Prochilodus scrofa. Ecotoxicol Environ Saf 52:83–91
Chamarthi RR, Bangeppagari M, Gooty JM, Mandala S, Tirado JO, Marigoudar SR (2014) Histopathological alterations in the gill, liver and brain of Cyprinus carpio on exposure to quinalphos. Am J Life Sci 2:211–216
Cinar K, Aksoy A, Emre Y, Aşti RN (2009) The histology and histochemical aspects of gills of the flower fish, Pseudophoxinus antalyae. Vet Res Commun 33:453–460
Crawshaw LI (1979) Responses to rapid temperature change in vertebrate ectotherms. Integr Comp Biol 19:225–237
Donaldson MR, Cooke SJ, Patterson DA, Macdonald JS (2008) Cold shock and fish. J Fish Biol 73:1491–1530
Dorval J, Leblond V, Deblois C, Hontela A (2005) Oxidative stress and endocrine endpoints in white sucker (Catostomus commersoni) from a river impacted by agricultural chemicals. Environ Toxicol Chem 24:1273–1280
Erhunmwunse NO, Ekaye SA, Ainerua MO, Ewere EE (2014) Histopathological changes in the brain tissue of Africa catfish exposure to glyphosate herbicide. J Appl Sci Environ Manage 18:275–280
Faheem M, Lone KP (2017) Oxidative stress and histopathologic biomarkers of exposure to bisphenol-A in the freshwater fish. Braz J Pharm Sci, Ctenopharyngodon idella. https://doi.org/10.1590/s2175-97902017000317003
Fanta E, Rios FSA, Romao S, Vianna ACC, Freiberger S (2003) Histopathology of the fish Corydoras paleatus contaminated with sublethal levels of organophosphorus in water and food. Ecotoxicol Environ Saf 54:119–130
Fernandes MN, Mazon AF (2003) Environmental pollution and fish gill morphology. In: Val AL, Kapoor BG (eds) Fish adaptations. Science Publishers, Enfield, pp 203–231
Gharred T, Naija A, Bouali RR, Haouas Z, Chénais B (2015) Assessment of oxidative stress and histopathological biomarkers in the Parablennius Incognitus fish as potential contamination indicators of the Bay of Sousse (Tunisia). J Marine Sci Res Dev 5:166
Halliwell B, Gutteridge JMC (1999) Free Radicals in Biology and Medicine. Oxford University Press, New York
Halliwell B, Gutteridge JMC (2007) Free Radicals in Biology and Medicine. Oxford University Press, New York
Hang HC, Bertozzi CR (2005) The chemistry and biology of mucin-type O-linked glycosylation. Bioorg Med Chem 13:5021–5034
Heise K, Puntarulo S, Nikinmaa M, Abele D, PÖrtner HO, (2006) Oxidative stress during stressful heat exposure and recovery in the North Sea eelpout Zoarces viviparus L. J Exp Biol 209:353–363
Hinton DE, Laurén DJ (1990) Liver structural alterations accompanying chronic toxicity in fishes: potential biomarkers of exposure. In: McCarthy JF, Shugart LR (eds) Biomarkers of Environmental Contamination. Lewis Publishers, Boca Raton, pp 51–65
Jezierska B, Witeska M (2004) The effect of metals on fish gill functions-Gas and ion change. Fresen Environ Bull 13:1370–1378
Joy S, Alikunju AP, Jose J, Sudha HSH, Parambath PM, Puthiyedathu ST, Philip B (2017) Oxidative stress and antioxidant defense responses of Etroplus suratensis to acute temperature fluctuations. J Therm Biol 70:20–26
Lakshmaiah G (2017) Brain histopathology of the fish Cyprinus carpio exposed to lethal concentrations of an organophosphate insecticide phorate. Int J Adv Res Dev 2:668–672
Ledy K, Giambérini L, Pihan JC (2003) Mucous cell responses in gill and skin of brown trout Salmo trutta fario in acidic, aluminium-containing stream water. Dis Aquat Organ 56:235–240
Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68:253–278
Liu Y, Ma D, Xiao Z, Xu S, Wang Y, Wang Y, Xiao Y, Song Z, Teng Z, Liu Q, Li J (2015) Histological change and heat shock protein 70 expression in different tissues of Japanese flounder Paralichthys olivaceus in response to elevated temperature. Chin J Oceanol Limn 33:11–19
Mallat J (1985) Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can J Fish Aquat Sci 42:630–648
Mansouri B, Maleki A, Johari SA, Shahmoradi B, Mohammadi E, Davari B (2017) Histopathological effects of copper oxide nanoparticles on the gill and intestine of common carp (Cyprinus carpio) in the presence of titanium dioxide nanoparticles. Chem Ecol 33:295–308
Martinez CB, Nagae MY, Zaia CTB, Zaia DMA (2004) Morphological and physiological acute effects of lead in the neotropical fish Prochilodus lineatus. Brazil J Biol 64:797–807
Mates JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104
McManus JFA (1948) Histological and histochemical uses of periodic acid. Stain Technol 23:99–108
Mekkawy IAA, Mahmoud UM, Wassif ET, Naguib M (2013) Effects of cadmium on some histological and histochemical characteristics of the kidney and gills tissues of Oreochromis niloticus (Linnaeus, 1758) dietary supplemented with tomato paste and vitamin E. J Fish Aquat Sci 8:553–580
Mishra A, Devi Y (2014) Histopathological alterations in the brain (optic tectum) of the fresh water teleost Channa punctatus in response to acute and subchronic exposure to the pesticide Chlorpyrifos. Acta Histochem 116:176–181
Mittal AK, Ueda T, Fujimori O, Yamada K (1994) Histochemical analysis of glycoproteins in the unicellular glands in the epidermis of an Indian freshwater fish Mastacembelus pancalus (Hamilton). Histochem J 26:666–677
Moron SE, de Andrade CA, Fernandes MN (2009) Response of mucous cells of the gills of traíra (Hoplias malabaricus) and jeju (Hoplerythrinus unitaeniatus) (Teleostei: Erythrinidae) to hypo- and hyper-osmotic ion stress. Neotrop Ichthyol 7:491–498
Mowry RW (1956) Alcian blue technics for the histochemical study of acidic carbohydrates. J Histochem Cytochem 4:407–408
Nelson DL, Cox MM (2008) Lehninger – Principles of Biochemistry, 5th edn. W.H, Freeman and Company
Oropesa-Jiménez AL, García-Cambero JP, Gómez-Gordo L, Roncero-Cordero V, Soler-Rodríguez F (2005) Gill modifications in the freshwater fish Cyprinus carpio after subchronic exposure to simazine. Bull Environ Contam Toxicol 74:785–792
Peixoto FP, Carrola J, Coimbra AM, Fernandes C, Teixeira P, Coelho L, Conceição I, Oliveira MM, Fontainhas-Fernandes A (2013) Oxidative stress responses and histological hepatic alterations in barbel, Barbus bocagei, from Vizela river, Portugal. Rev Int Contam Ambie 29:29–38
Pelster B, Wood CM, Jung E, Val AL (2018) Air-breathing behavior, oxygen concentrations, and ROS defense in the swim bladders of two erythrinid fish, the facultative air-breathing jeju, and the non-air-breathing traira during normoxia, hypoxia and hyperoxia. J Comp Physiol B 188:437–449
Philippart JC, Ruwet JC (1982) Ecology and distribution of tilapias. In: Pullin RSV, Lowe-McConnell RH (eds) The biology and culture of tilapias. ICLARM Conf Proc 7, pp 15–60
Poleksic V, Mitrovic-Tutundzic V (1994) Fish gills as a monitor of sublethal and chronic effects of pollution. In: Müller R, Lloyd R (eds) Sublethal and Chronic effects of pollutants on freshwater fish. Fishing News Books, Oxford, pp 339–352
Powell MD (2007) Respiration in infectious and non-infectious gill diseases. In: Fernandes MN, Glass ML, Rantin FT, Kapoor BG (eds) Fish Respiration and Environment. Science Publisher, Enfield, pp 317–339
Roberts SD, Powell MD (2003) Comparative ionic flux and gill mucous cell histochemistry: effects of salinity and disease status in Atlantic salmon (Salmo salar L.). Com Biochem Physiol A 134:525–537
Ruas CBG, Carvalho CS, de Araújo HSS, Espíndola ELG, Fernandes MN (2008) Oxidative stress biomarkers of exposure in the blood of Cichlid species from a metal-contaminated river. Ecotoxicol Environ Saf 71:86–93
Saber TH (2011) Histological adaptation to thermal changes in gills of common carp fishes Cyprinus carpio L. J Raf Sci 22:46–55
Sarasquete C, Gisbert E, Ribeiro L, Vieira L, Dinis MT (2001) Glycoconjugates in epidermal, branchial, and digestive mucous cells and gastric glands of gilthead sea bream, Sparus aurata, Senegal sole, Solea senegalensis, and Siberian sturgeon, Acipenser baeri development. Eur J Histochem 45:267–278
Sibbing FA, Uribe R (1985) Regional specialization in the oropharyngeal wall and food processing in the carp Cyprinus carpio. Neth J Zool 35:377–422
Somero GN (2005) Linking biogeography to physiology: evolutionary and acclamatory adjustments of thermal limits. Front Zool 2:1–9
Srivastava N, Kumari U, Rai AK, Mittal S, Mittal AK (2012) Histochemical analysis of glycoproteins in the gill epithelium of an Indian major carp, Cirrhinus mrigala. Acta histochem 114:626–635
Tabarraei H, Hassan J, Mosavi SS (2019) Determination of LD50 of some essential oils and histopathological changes in short-term exposure to one of them in rainbow trout (Oncorhynchus mykiss). Toxicol Res Application 3:1–7
Takashima F, Hibiya T (1995) An atlas of fish histology: Normal and pathological features. Gustav Fischer Verlag, Kodanska, Tokyo
Teh SJ, Adams SM, Hinton DE (1997) Histopathologic biomarkers in feral freshwater fish populations exposed to different types of contaminant stress. Aquat Toxicol 37:51–70
Temmink J, Bowmieister P, Jong P, Van der Berg J (1983) An ultrastructural study of chromate-induced hyperplasia in the gill of rainbow trout, Salmo gairdneri. Aquat Toxicol 4:165–179
Thomas RE, Gharrett JA, Carls MG, Rice SD, Moles A, Korn S (1986) Effects of fluctuating temperature on mortality, stress, and energy reserves of juvenile coho salmon. Trans Am Fish Soc 115:52–59
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharm 13:57–149
Vigliano FA, Aleman N, Quiroga MI, Nieto JM (2006) Ultrastructural characterization of gills in the juveniles of the Argentinian Silverside, Odontesthes bonariensis (Valenciennes, 1835) (Teleostei: Atheriniformes). Anat Histol Embryol 35:76–83
Wilkie MP, Wood CM (1994) The effects of extremely alkaline water (pH 9.5) on rainbow trout gill function and morphology. J Fish Biol 45:87–98
Winkaler EU, Silva AG, Galindo HC, Martinez CBR (2001) Biomarcadores histológicos e fisiológicos para o monitoramento da saúde de peixes de ribeirões de Londrina, Estado do Paraná. Acta Scient Biol Sci 23:507–514
Wu SM, Liu JH, Shu LH, Chen CH (2015) Anti-oxidative responses of zebrafish (Danio rerio) gill, liver and brain tissues upon acute cold shock. Comp Biochem Physiol A Mol Integr Physiol 187:202–213
Xie S, Zheng K, Chen J, Zhang Z, Zhu X, Yang Y (2011) Effect of water temperature on energy budget of Nile tilapia, Oreochromis niloticus. Aquacult Nutr 17:683–690
Acknowledgements
This manuscript was proofread by Kevin Kavanagh at E.C.C. Language School, Chiang Mai, Thailand. This research was supported by a Science Achievement Scholarship of Thailand, the Graduate Master’s Degree Program in Biology, Faculty of Science, Chiang Mai University, Chiang Mai, 50200, Thailand, the Biodiversity–Based Economy Development Office [BEDO], (public organization), under the research program: Climate Change Impact Assessment on Ecological System and Environment in Kwan Phayao for Adaptation (research grant number, R59111), the Division of Fisheries, School of Agriculture and Natural Resources, University of Phayao, and the Unit of Excellence 2020 on Biodiversity and Natural Resources Management, University of Phayao.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All fish procedures were approved by committees of the Institutional Animal Care, University of Phayao, Thailand (ID: 5901040011) following the guidelines given by the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Phrompanya, P., Panase, P., Saenphet, S. et al. Histopathology and oxidative stress responses of Nile tilapia Oreochromis niloticus exposed to temperature shocks. Fish Sci 87, 491–502 (2021). https://doi.org/10.1007/s12562-021-01511-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-021-01511-y