Abstract
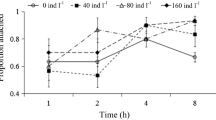
There are two types of movement pattern in the Brachionidae (rotifers), swimming and attachment, although the factors that induce a shift between them have not been adequately clarified. This study investigated the effects of five external factors—food limitation, temperature, salinity, predator, and un-ionized ammonia—on movement in females of the euryhaline rotifer Brachionus plicatilis sensu stricto. Rotifers under periodic starvation showed increased swimming frequency (percentage of swimming rotifers) to about 50% on feeding compared to controls under continuous feeding (21%). Starved rotifers were further exposed to the following conditions for 2 days: a range of water temperature (15–25 °C), salinity (17–34 practical salinity units), a predator (or other rotifers)-conditioned medium, and un-ionized ammonia (NH3-N; 5–20 mg/l). Neither temperature nor predator conditioning significantly affected rotifer swimming frequency. However, rotifers transferred to a higher salinity or to a rotifer-conditioned medium ceased to exhibit swimming. All the tested ammonia levels caused vigorous swimming of rotifers during the initial experimental period. The results indicate that swimming in female rotifers can be classified as (1) an escape behavior induced by environmental stress, and (2) a response to higher viability under certain favorable environmental conditions.



Similar content being viewed by others
References
Alver MO, Hagiwara A (2007) An individual-based population model for the prediction of rotifer population dynamics and resting egg production. Hydrobiologia 593:19–26
Araujo A, Hagiwara A (2005) Screening methods for improving rotifer culture quality. Hydrobiologia 546:553–558
Arauzo M (2003) Harmful effects of un-ionized ammonia on the zooplankton community in a deep waste treatment pond. Water Res 37:1048–1054
Batchelder HP, Edwards CA, Powell TM (2002) Individual-based models of copepod populations in coastal upwelling regions: implications of physiologically and environmentally influenced diel vertical migration on demographic success and nearshore retention. Prog Oceanogr 53:307–333
Bower CE, Bidwell JP (1978) Ionization of ammonia in seawater: effects of temperature, pH, salinity. J Fish Res Board Can 35:1012–1016
Buskey EJ, Coulter C, Strom S (1993) Locomotory patterns of microzooplankton: potential effects on food selectivity of larval fish. Bull Mar Sci 53:29–43
Charoy C, Clément P (1993) Foraging behavior of Brachionus calyciflorus (Pallas): variations in the swimming path according to presence or absence of algal food (Chlorella). Hydrobiologia 255:95–100
Charoy C, Janssen CR (1999) The swimming behavior of Brachionus calyciflorus (rotifer) under toxic stress. Chemosphere 38:3247–3260
Clément P (1987) Movements in rotifers: correlations of ultrastructure and behavior. In: May L, Wallace R, Herzing A (eds) Rotifer symposium IV. Developments in Hydrobiologia, vol. 42. Springer, Dordrecht, 339−359
Cobcroft JM, Pankhurst PM (2006) Visual field of cultured striped trumpeter Latris lineata (Teleostei) larvae feeding on rotifer prey. Mar Freshwater Behav Physiol 39:193–208
Coughlin DJ (1993) Pre-locating by clownfish (Amphiprion perideraion) larvae feeding on rotifers (Brachionus plicatilis). J Plankton Res 15:117–123
Dahms H-U, Hagiwara A, Lee J-S (2011) Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquat Toxicol 101:1–12
de Araujo AB, Snell TW, Hagiwara A (2000) Effect of unionized ammonia, viscosity and protozoan contamination on the enzyme activity of the rotifer Brachionus plicatilis. Aquac Res 31:359–365
de Araujo AB, Hagiwara A, Snell TW (2001) Effect of unionized ammonia, viscosity and protozoan contamination on reproduction and enzyme activity of the rotifer Brachionus rotundiformis. Hydrobiology 446:363–368
Epp RW, Lewis WM (1984) Cost and speed of locomotion for rotifer. Oecologia 61:289–292
Fielder DS, Purser GJ, Battaglene SC (2000) Effect of rapid changes in temperature and salinity on availability of the rotifer Brachionus rotundiformis and Brachionus plicatilis. Aquaculture 189:85–99
Gilbert JJ (2011) Temperature, kairomones and phenotypic plasticity in rotifer Keratella tropica (Apstein, 1907). Hydrobiologia 678:179–190
Gilbert JJ (2014) Morphological and behavioral responses of a rotifer to the predator Asplanchna. J Plankton Res 36:1576–1584
Gilbert JJ, Starkweather PL (1978) Feeding in the rotifer Brachionus calyciflorus. III. Direct observations on the effect of food type, food density, change in food type, and starvation on the incidence of pseudotrochal screening. Int Ver Theor Angew Limnol Verhandl 20:2382–2388
Gómez A, Carmona MJ, Serra M (1997) Ecological factors affecting gene flow in the Brachionus plicatilis complex (Rotifera). Oecologia 111:350–356
Grageda AC, Sakakura Y, Minamimoto M, Hagiwara A (2005) Differences in life-history traits in two clonal strains of the self-fertilized fish, Rivulus marmoratus. Environ Biol Fishes 73:427–436
Hagiwara A, Hino A, Hirano R (1988) Effects of temperature and chlorinity on resting egg formation in the rotifer Brachionus plicatilis. Nippon Suisan Gakk 54:569–575
Hagiwara A, Lee C-S, Miyamoto G, Hino A (1989) Resting egg formation and hatching of the S-type rotifer Brachionus plicatilis at varying salinities. Mar Bio 103:327–332
Hagiwara A, Hamada K, Nishi A, Imaizumi K, Hirayama K (1993) Mass production of rotifer Brachionus plicatilis resting egg in 50 m3 tanks. Nippon Suisan Gakk 59:93–98
Hagiwara A, Hamada K, Hori S, Hirayama K (1994) Increased sexual reproduction in Brachionus plicatilis (Rotifera) with the addition of bacterial and rotifer extracts. J Exp Mar Bio Ecol 181:1–8
Hagiwara A, Yamamiya N, de Araujo AB (1998) Effect of water viscosity on the population growth of the rotifer Brachionus plicatilis Müller. Hydrobiologia 387(388):489–494
Hagiwara A, Gallardo WG, Assavaaree M, Kotani T, de Araujo AB (2001) Live food production in Japan: recent progress and future aspects. Aquaculture 200:111–127
Hagiwara A, Kim H-J, Marcial H (2017) Mass culture and preservation of Brachionus plicatilis sp. Complex. In: Hagiwara A, Yoshinaga T (eds) Rotifers, Fisheries Science Series. Springer Nature, Singapore, pp 35–46
Han C, Kim H-J, Suga K, Li M, Hagiwara A (2018) Comparison of resting egg gene expression with different hatchability related to salinity variation in the marine rotifer Brachionus manjavacas. Fish Sci 84:663–669
Hirayama K, Kusano T (1972) Fundamental studies on physiology of rotifer for its mass culture. II. Influence of water temperature on population growth of rotifer. Nippon Suisan Gakk 38:1357–1363
Janssen CR, Ferrando MD, Persoone G (1993) Ecotoxicological studies with the freshwater rotifer Brachionus calyciflorus. I. Conceptual framework and applications. Hydrobiologia 255(256):21–32
Janssen CR, Ferrando MD, Persoone G (1994) Ecotoxicological studies with the freshwater rotifer Brachionus calyciflorus. VI. Rotifer behavior as a sensitive and rapid sublethal test criterion. Ecotoxicol Environ Saf 28:244–255
Kim H-J, Hagiwara A (2011) Effect of salinity during resting egg formation and hatching on descendent reproduction in the rotifer Brachionus rotundiformis Tschugunoff. J Plankton Res 33:1033–1042
Kim H-J, Suga K, Hagiwara A (2013) Effect of light wavelength on the sexual and asexual reproduction of the monogonont rotifer Brachionus manjavacas. Aquacult Sci 61:261–268
Kim H-J, Lee J-S, Hagiwara A (2018) Phototactic behavior of live food rotifer Brachionus plicatilis species complex and its significance in larviculture: a review. Aquaculture 497:253–259
Lass S, Spaak P (2003) Chemically induced anti-predator defenses in plankton: a review. Hydrobiologia 491:221–239
Loose CJ, Dawidowicz P (1994) Trade-offs in diel vertical migration by zooplankton: the costs of predator avoidance. Ecology 75:2255–2263
Lowe CD, Kemp SL, Bates AD, Montagnes DJS (2005) Evidence that the rotifer Brachionus plicatilis is not an osmoconformer. Mar Biol 146:923–929
Luciani A, Chassé JL, Clément P (1983) Aging in Brachionus plicatilis: the evolution of swimming as a function of age at two different calcium concentrations. Hydrobiologia 104:141–146
Mills S, Alcántara-Rodríguez JA, Ciros-Pérez J, Gómez A, Hagiwara A, Galindo KH, Jersabek CD, Malekzadeh-Viayeh R, Leasi F, Lee J-S, Welch DBM, Papakostas S, Riss S, Segers H, Serra M, Shiel R, Smolak R, Snell TW, Stelzer C-P, Tang CQ, Wallace RL, Fontaneto D, Walsh EJ (2017) Fifteen species in one: deciphering the Brachionus plicatilis species complex (Rotifera, Monogononta) through DNA taxonomy. Hydrobiologia 796:39–58
Preston BL, Cecchine G, Snell TW (1998) Effects of pentachlorophenol on predator avoidance behavior of the rotifer Brachionus calyciflorus. Aquat Toxicol 44:201–212
Preston BL, Snell TW, Dusenbery DB (1999a) The effects of sublethal pentachlorophenol exposure on predation risk in freshwater rotifer species. Aquat Toxicol 47:93–105
Preston BL, Snell TW, Kneisel R (1999b) UV-B exposure increases acute toxicity of pentachlorophenol and mercury to the rotifer Brachionus calyciflorus. Environ Pollut 106:23–31
Réale D, Clément P, Esparcia-Collado A (1993) Influence of the concentration of oxygen on the swimming path of Brachionus plicatilis (Rotifera). Hydrobiology 255(256):87–93
Rhee J-S, Kim R-O, Choi H-G, Lee J, Lee Y-M, Lee J-S (2011) Molecular and biochemical modulation of heat shock protein 20 (Hsp20) gene by temperature stress and hydrogen peroxide (H2O2) in the monogonont rotifer, Brachionus sp. CBPC 154:19–27
SakakuraY Noakes DLG (2000) Age, growth, and sexual development in the self-fertilizing hermaphroditic fish Rivulus marmoratus. Environ Biol Fishes 59:309–371
Sarma SSS, Resendiz RAL, Nandini S (2011) Morphometric and demographic responses of brachionids prey (Brachionus calyciflorus Pallas and Plationus macracanthus (Daday)) in the presence of different densities of the predator Asplanchna brightwellii (Rotifera: Asplanchnidae). Hydrobiologia 662:179–187
Seuront L, Yamazaki H, Souissi S (2004) Hydrodynamic disturbance and zooplankton swimming behavior. Zool Stud 43:376–387
Sheikh AA, Khursheed I, Ahmad MJ, Ahad I, Tali FA, Nabi SU (2017) Role of infochemicals to enhance the efficacy of biocontrol agents in pest management. Int J Chem Stud 5:655–662
Snell TW (1998) Chemical ecology of rotifers. Hydrobiologia 387(388):267–276
Snell TW, Childress M (1987) Aging and loss of fertility in male and female Brachionus plicatilis (Rotifera). Int J Invertebr Repr Dev 12:103–110
Snell TW, Hoff FH (1987) Fertilization and male fertility in the rotifer Brachionus plicatilis. Hydrobiologia 147:329–334
Snell TW, Childress MJ, Boyer EM, Hoff FH (1987) Assessing the status of rotifer mass cultures. J World Aquacult Soc 18:270–277
Vadstein O, Olsen LM, Andersen T (2012) Prey-predator dynamics in rotifer: density-dependent consequences of spatial heterogeneity due to surface attachment. Ecology 93:1795–1801
Vet L, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Wallace RL (1980) Ecology of sessile rotifers. Hydrobiologia 73:181–193
Wallace RL, Smith HA (2013) Rotifera. Wiley, Chichester
Yoshinaga T, Hagiwara A, Tsukamoto K (1999) Effect of conditioned media on the asexual reproduction of the monogonont rotifer Brachionus plicatilis O. F. Müller. Hydrobiologia 412:103–110
Yoshinaga T, Hagiwara A, Tsukamoto K (2003) Life history response and age-specific tolerance to starvation in Brachionus plicatilis O. F. Müller (Rotifera). J Exp Mar Biol Ecol 287:261–271
Yu J-P, Hirayama K (1986) The effect of un-ionized ammonia on the population growth of the rotifer in mass culture. Nippon Suisan Gakk 52:1509–1513
Yúfera M (2007) Swimming behavior of Brachionus plicatilis in relation to food concentration and feeding rates. Hydrobiologia 593:13–18
Yúfera M, Pascual E, Olivares JM (2005) Factors affecting swimming speed in the rotifer Brachionus plicatilis. Hydrobiologia 546:375–380
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice hall, Englewood Cliffs, pp 563–564
Acknowledgments
This research was supported by a Ministry of Education, Culture, Sports, Science and Technology Grant-in-Aid for Scientific Research (B) (2012–2014, no. 24380108; 2017–2019, no. 17H03862) to A. H., and research fellowships for young researchers (2019–2021, no. 19K15897) to H.-J. K. The authors deeply appreciate the valuable comments of Prof. T. W. Snell of the Georgia Institute of Technology and Dr. Robert Nesta Kagali at Nagasaki University, as well as those of the many anonymous contributors to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, HJ., Ohtani, M., Kakumu, A. et al. External factors that regulate movement in the marine rotifer Brachionus plicatilis. Fish Sci 86, 655–663 (2020). https://doi.org/10.1007/s12562-020-01438-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-020-01438-w