Abstract
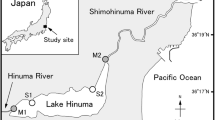
To clarify the role of salt marsh creeks as fish refuges, predation risks for two small species (the nektonic Oryzias latipes and benthic Acanthogobius lactipes) were compared among three microhabitats (upper and lower areas of a creek, and marsh edge separate from the creek) in a salt marsh in Lake Hinuma, eastern Japan, in July 2016, using daytime tethering experiments. The survival rate of O. latipes was highest in the upper creek (96%) and lowest at the marsh edge (62%), whereas no significant differences were found among the microhabitats for A. lactipes, with high survival rates (> 90%) in all microhabitats. Individual numbers of larger piscivorous fishes, determined by fyke net sampling, were greatest at the marsh edge, whereas no individuals were recorded in the upper creek, which was characterized by shallower depths and lower dissolved oxygen levels. The results suggested that the upper creek provides potential refugia for O. latipes, which occupied the upper and middle layers of the water column and exhibited hypoxia tolerance. The higher survival rate of benthic A. lactipes at the marsh edge may be due to their cryptic coloration in relation to bottom sediments, which helps them to avoid predation.


Similar content being viewed by others
References
Aronson RB, Heck KL (1995) Tethering experiments and hypothesis testing in ecology. Mar Ecol Prog Ser 121:307–309
Banikas EM, Thompson JS (2012) Predation risk experienced by mummichog, Fundulus heteroclitus, in intertidal and subtidal salt marsh habitats. Estuar Coast 35:1346–1352
Bretsch K, Allen DM (2006) Tidal migrations of nekton in salt marsh intertidal creeks. Estuar Coast 29:474–486
Cattrijsse A, Hampel H (2006) European intertidal marshes: a review of their habitat functioning and value for aquatic organisms. Mar Ecol Prog Ser 324:293–307
Corman SS, Roman CT (2011) Comparison of salt marsh creeks and ditches as habitat for nekton. Mar Ecol Prog Ser 434:57–66
Deegan LA, Hughes JE, Rountree RA (2000) Salt marsh ecosystem support of marine transient species. In: Weinstein MP, Kreeger DA (eds) Concepts and controversies in tidal marsh ecology. Kluwer, Dordrecht, pp 333–365
Desmond JS, Zedler JB, Williams GD (2000) Fish use of tidal creek habitats in two southern California salt marshes. Ecol Eng 14:233–252
Green BC, Smith DJ, Earley SE, Hepburn LJ, Underwood GJC (2009) Seasonal changes in community composition and trophic structure of fish populations of five salt marshes along the Essex coastline, United Kingdom. Estuar Coast Shelf Sci 85:247–256
Hackney CT, Burbanck WD, Hackney OP (1976) Biological and physical dynamics of a Georgia tidal creek. Chesapeake Sci 17:271–280
Halpin PM (1997) Habitat use patterns of the mummichog, Fundulus heteroclitus, in New England. I. Intramarsh variation. Estuaries 20:618–625
Halpin PM (2000) Habitat use by an intertidal salt-marsh fish: tradeoffs between predation and growth. Mar Ecol Prog Ser 198:203–214
Hammerschlag N, Morgan AB, Serafy JE (2010) Relative predation risk for fishes along a subtropical mangrove-seagrass ecotone. Mar Ecol Prog Ser 401:259–267
Hecht T, van der Lingen CD (1992) Turbidity-induced changes in feeding strategies of fish in estuaries. S Afr J Zool 27:95–107
Heck KL, Thoman TA (1981) Experiments on predator-prey interactions in vegetated aquatic habitats. J Exp Mar Biol Ecol 53:125–134
Horinouchi M (2007) Distribution patterns of benthic juvenile gobies in and around seagrass hanitats: effectiveness of seagrass shelter against predators. Estuar Coast Shelf Sci 72:657–664
Igari K, Endou T, Kaneko S, Usui S, Kanou K (2015) Seasonal dynamics of fishes collected by small fyke nets in reed belts in Lake Kitaura, Ibaraki Prefecture, Japan. Bull Biogeogr Soc Jpn 70:113–122 (in Japanese with English abstract)
Jin B, Fu C, Zhong J, Li B, Chen J, Wu J (2007) Fish utilization of a salt marsh intertidal creek in the Yangtze River estuary, China. Estuar Coast Shelf Sci 73:844–852
Kaneko S, Kanou K, Sano M (2016) Food habits of salt marsh fishes in Lake Hinuma, Ibaraki Prefecture, central Japan. Fish Sci 82:631–637
Kaneko S, Kanou K, Sano M (2019) Comparison of fish assemblage structures among microhabitats in a salt marsh in Lake Hinuma, eastern Japan. Fish Sci 85:113–125
Kanou K, Sano M, Kohno H (2004) Food habits of fishes on unvegetated tidal mudflats in Tokyo Bay, central Japan. Fish Sci 70:978–987
Kneib RT (1997) The role of tidal marshes in the ecology of estuarine nekton. Oceanogr Mar Biol Annu Rev 35:163–220
Kouzuki Y, Sato Y, Murakami H, Nishioka K, Kurata K, Saraie K, Hukuta M (2000) Environmental factors of Medaka Oryzias latipes in suburban irrigation canal. Environ Syst Res 28:313–320 (in Japanese with English abstract)
Laffaille P, Feunteun E, Lefeuvre JC (2000) Composition of fish communities in a European macrotidal salt marsh (the Mont Saint-Michel Bay, France). Estuar Coast Shelf Sci 51:429–438
Linehan JE, Gregory RS, Schneider DC (2001) Predation risk of age-0 cod (Gadus) relative to depth and substrate in coastal waters. J Exp Mar Biol Ecol 263:25–44
Mathieson S, Cattrijsse A, Costa MJ, Drake P, Elliott M, Gardner J, Marchand J (2000) Fish assemblages of European tidal marshes: a comparison based on species, families and functional guilds. Mar Ecol Prog Ser 204:225–242
Mclvor CC, Odum WE (1988) Food, predation risk, and microhabitat selection in a marsh fish assemblage. Ecology 69:1341–1351
Ministry of the Environment of Japan (2015) Red data book 2014; brackish and freshwater fishes. Gyosei, Tokyo (in Japanese)
Morton RM, Pollock BR, Beumer JP (1987) The occurrence and diet of fishes in a tidal inlet to a saltmarsh in southern Moreton Bay, Queensland. Aust J Ecol 12:217–237
Nakabo T (2013) Fishes of Japan with pictorial keys to the species, 3rd edn. Tokai University Press, Hadano (in Japanese)
Nakamura Y, Sano M (2004) Is there really lower predation risk for juvenile fishes in a seagrass bed compared with an adjacent coral area? Bull Mar Sci 74:477–482
Nakamura T, Katano O, Yamamoto S (2004) Effects of aquatic plant zones on the reduction of predation pressure on Japanese native fish by smallmouth bass Micropterus dolomieu. Suisanzoshoku 52:287–291 (in Japanese with English abstract)
Nakane Y, Suda Y, Hayakawa Y, Ohtomi J, Sano M (2009) Predation pressure for a juvenile fish on an exposed sandy beach: comparison among beach types using tethering experiments. La mer 46:109–115
Nakane Y, Suda Y, Sano M (2011) Food habits of fishes on an exposed sandy beach at Fukiagehama, South-West Kyushu Island, Japan. Helgol Mar Res 65:123–131
Nanjo K, Nakamura Y, Horinouchi M, Kohno H, Sano M (2011) Predation risks for juvenile fishes in a mangrove estuary: a comparison of vegetated and unvegetated microhabitats by tethering experiments. J Exp Mar Biol Ecol 405:53–58
Paterson AW, Whitfield AK (2000) Do shallow-water habitats function as refugia for juvenile fishes? Estuar Coast Shelf Sci 51:359–364
Paterson AW, Whitfield AK (2003) The fishes associated with three intertidal salt marsh creeks in a temperate southern African estuary. Wetl Ecol Manage 11:305–315
Peterson CH, Black R (1994) An experimentalist’s challenge: when artifacts of intervention interact with treatments. Mar Ecol Prog Ser 111:289–297
Reid SM, Fox MG, Whillans TH (1999) Influence of turbidity on piscivory in largemouth bass (Micropterus salmoides). Can J Fish Aquat Sci 56:1362–1369
Rountree RA, Able KW (1992) Fauna of polyhaline subtidal marsh creeks in southern New Jersey: composition, abundance and biomass. Estuaries 15:171–185
Rountree RA, Able KW (1997) Nocturnal fish use of New Jersey marsh creek and adjacent bay shoal habitats. Estuar Coast Shelf Sci 44:703–711
Rozas LP, Hackney CT (1984) Use of oligohaline marshes by fishes and macrofaunal crustaceans in North Carolina. Estuaries 7:213–224
Ruiz GM, Hines AH, Posey MH (1993) Shallow water as a refuge habitat for fish and crustaceans in non-vegetated estuaries: an example from Chesapeake Bay. Mar Ecol Prog Ser 99:1–16
Rypel AL, Layman CA, Arrington DA (2007) Water depth modifies relative predation risk for a motile fish taxon in Bahamian tidal creeks. Estuar Coast 30:518–525
Salgado J, Costa MJ, Cabral H, Deegan L (2004) Comparison of the fish assemblages in tidal salt marsh creeks and in adjoining mudflat areas in the Tejo estuary (Portugal). Cah Biol Mar 45:213–224
Savino JF, Stein RA (1982) Predator-prey interaction between largemouth bass and bluegills as influenced by simulated, submersed vegetation. Trans Am Fish Soc 111:255–266
Sogard SM (1992) Variability in growth rates of juvenile fishes in different estuarine habitats. Mar Ecol Prog Ser 85:35–53
Acknowledgements
We are grateful to Yuichi Tanaka and Shun Kawaida for helpful suggestions on the statistical analyses. Our thanks are also due to Ken Okamoto, Shigeru Aoki and the anonymous reviewers for constructive comments on the manuscript, and to Graham Hardy for reviewing the English. We are indebted to the Ohinuma Fishermen’s Cooperative Association for permission to undertake sampling in Lake Hinuma. This study complied with Japanese law.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaneko, S., Kanou, K. & Sano, M. Comparison of predation risks for small fishes in salt marsh microhabitats in Lake Hinuma, eastern Japan, using tethering experiments. Fish Sci 85, 457–463 (2019). https://doi.org/10.1007/s12562-019-01296-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-019-01296-1