Abstract
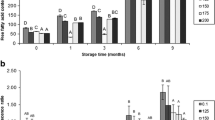
We investigated factors that accelerate dimethylamine formation in gadoid dark muscle. The degradation rate of trimethylamine-N-oxide into dimethylamine and formaldehyde during frozen storage was compared between ordinary muscle and dark muscle from walleye pollock, southern blue whiting, and hoki. Dimethylamine was generated faster in dark muscle than in ordinary muscle in each species, and it was produced most abundantly in hoki dark muscle compared with the other two species. We investigated the mechanism that caused dimethylamine to be generated more abundantly in dark muscle during frozen storage, and found that the amount of dark muscle nonheme iron, which catalyzes trimethylamine-N-oxide degradation, was higher than that in ordinary muscle in each species, and hoki dark muscle in particular contained the highest levels of nonheme iron among these three species. Moreover, dark muscle in all three fish species had a higher taurine content (known to accelerate dimethylamine formation) than ordinary muscle. These data suggested two candidate factors, namely nonheme iron and taurine, that may accelerate dimethylamine generation during frozen storage. In addition, gel filtration results suggested that walleye pollock dark muscle contains as yet unidentified low-molecular-weight agents that stably accelerate dimethylamine generation.








Similar content being viewed by others
References
Reppond KD, Collins J, Markey D (1985) Walleye pollock (Theragra chalcogramma): changes in quality when held in ice, slush-ice, refrigerated seawater, and CO2-modified refrigerated seawater then stored as blocks of fillets at −18°C. J Food Sci 50:985–996
Tokunaga T (1974) The effect of decomposed products of trimethylamine oxide on quality of frozen Alaska pollack fillet (in Japanese with English abstract). Nippon Suisan Gakkaishi 40:167–174
Castell CH, Smith B, Dyer WJ (1973) Effect of formaldehyde on salt extractable proteins of gadoid muscle. J Fish Res Board Can 30:1205–1213
Dyer WJ (1952) Amines in fish muscle. VI. Trimethylamine oxide content of fish and marine invertebrates. J Fish Res Board Can 8:314–324
Tokunaga T (1970) Trimethylamine oxide and its decomposition in the bloody muscle of fish. I. TMAO, TMA, and DMA contents in ordinary and bloody muscles (in Japanese with English abstract). Nippon Suisan Gakkaishi 36:502–509
Castell CH, Neal WE, Dale J (1973) Comparison of changes in trimethylamine, dimethylamine, and extractable protein in iced and frozen gadoid fillets. J Fish Res Board Can 30:1246–1248
Monkovic I, Wong H, Bachand C (1985) Secondary amines from iron(II) ion-catalyzed reaction of amine oxides: a general method for the dealkylation of tertiary amines. Synthesis 8:770–773
Spinelli J, Koury B (1979) Nonenzymic formation of dimethylamine in dried fishery products. J Agric Food Chem 27:1104–1108
Sotelo CG, Pineiro C, Perez-Martin RI (1995) Denaturation of fish proteins during frozen storage: role of formaldehyde (in English with German abstract). Z Lebensm Unters Forsch 200:14–23
Amano K, Yamada K (1964) A biological formation of formaldehyde in the muscle of gadoid fish. Nippon Suisan Gakkaishi 30:430–435
Kimura M, Seki N, Kimura I (2000) Occurrence and some properties of trimethylamine-N-oxide demethylase in myofibrillar fraction from walleye pollack muscle. Fish Sci 66:725–729
Tokunaga T (1980) Biochemical and food scientific study on trimethylamine oxide and its related substances in marine fishes (in Japanese with English abstract). Bull Tokai Reg Fish Res Lab 101:1–129
Rehbein H (1988) Relevance of trimethylamine oxide demethylase activity and haemoglobin content to formaldehyde production and texture deterioration in frozen stored minced fish muscle. J Sci Food Agric 43:261–276
Spinelli J, Koury BJ (1981) Some new observations on the pathways of formation of dimethylamine in fish muscle and liver. J Agric Food Chem 29:327–331
Dingle JR, Hines JA (1975) Protein instability in minced flesh from fillets and frames of several commercial Atlantic fishes during storage at −5°C. J Fish Res Board Can 32:775–783
Reece P (1983) The role of oxygen in the production of formaldehyde in frozen minced cod muscle. J Sci Food Agric 34:1108–1112
Castell CH, Smith B, Neal W (1971) Production of dimethylamine in muscle of several species of gadoid fish during frozen storage, especially in relation to presence of dark muscle. J Fish Res Board Can 28:1–5
Tokunaga T (1970) Trimethylamine oxide and its decomposition in the bloody muscle of fish. II. Formation of DMA and TMA during storage (in Japanese with English abstract). Nippon Suisan Gakkaishi 36:510–515
Obatake A (1988) Food chemical studies on the dark muscle of fish (in Japanese with English abstract). Bull Fac Agric Kochi Univ 52:79–157
Hashimoto Y, Okaichi T (1957) On the determination of trimethylamine and trimethylamine oxide. A modification of the Dyer method (in Japanese with English abstract). Nippon Suisan Gakkaishi 23:269–272
Dyer WJ, Dyer FE, Snow MJ (1952) Amines in fish muscle. V. Trimethylamine oxide estimation. J Fish Res Board Can 8:309–313
Dyer WJ, Mounsey YA (1945) Amines in fish muscle. II. Development of trimethylamine and other amines. J Fish Res Board Can 6:359–367
William H (ed) (2005) Chap. 4.6.01, Method 962.09. In: Official methods of analysis of the Association of Official Analytical Chemists, 18th edn. Association of Official Analytical Chemists, Gaithersburg, p 45
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Rhee KS, Ziprin YA (1987) Modification of the Schricker nonheme iron method to minimize pigment effects for red meats. J Food Sci 52:1174–1176
Kitahara Y, Matsuoka A, Kobayashi N, Shikama K (1990) Autoxidation of myoglobin from bigeye tuna fish (Thunnus obesus). Biochim Biophys Acta 1038:23–28
Duve CD (1948) A spectrophotometric method for the simultaneous determination of myoglobin and hemoglobin in extracts of human muscle. Acta Chem Scand 2:264–289
Tokunaga T (1965) Studies on the development of dimethylamine and formaldehyde in Alaska pollack muscle during frozen storage—II (in Japanese with English abstract). Bull Hokkaido Reg Fish Res Lab 30:90–97
Tokunaga T (1964) Studies on the development of dimethylamine and formaldehyde in Alaska pollack muscle during frozen storage (in Japanese with English abstract). Bull Hokkaido Reg Fish Res Lab 29:108–122
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mizuguchi, T., Kumazawa, K., Yamashita, S. et al. Factors that accelerate dimethylamine formation in dark muscle of three gadoid species during frozen storage. Fish Sci 77, 143–149 (2011). https://doi.org/10.1007/s12562-010-0303-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-010-0303-x