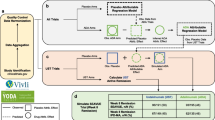
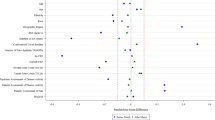
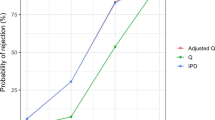
Abstract
Real-world (RW) data have been a source for creating external control arms to evaluate results from randomized controlled trials (RCTs) in rare diseases and scenarios where randomization to a control group is unethical or unfeasible. However, the validity of any decision making based on such comparative results depends heavily on the appropriateness and quality of the control arm data. FDA guidance lists multiple bias-generating concerns with the use of real-world controls arising from data quality and validity issues, which we frame as a data source ignorability assumption under the potential outcome framework. Hybrid control designs, RCTs with a full treatment group and a small underpowered control group supplemented with RW control data, have the potential to address some of these bias concerns. Statistical methods have been proposed for the analysis of hybrid designs and can adjust for potential violations of the data source ignorability assumption. A simulation study is presented to evaluate the operating characteristics of single and hybrid real-world control methods across the bias-generating scenarios mentioned in FDA guidance. Results suggest that certain methods can adjust for potential biases under these scenarios but may result in reduced efficiency through larger standard errors, or type I error inflation. Implications for the use of such methods and suggestions for additional work are discussed.





Similar content being viewed by others
References
Food and Drug Administration (2019) Demonstrating substantial evidence of effectiveness for human drug and biological products. https://www.fda.gov/media/133660/download. Accessed 17 Feb 2021
Silverman B (2018) A baker’s dozen of US FDA efficacy approvals using real world evidence. Pharma Intelligence Pink Sheet
Food and Drug Administration (2019) Rare diseases: Natural history studies for drug development. https://www.fda.gov/media/122425/download. Accessed 17 Feb 2021
Food and Drug Administration (2014) Blinatumomab drug approval package. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125557Orig1s000MedRedt.pdf. Accessed 17 Feb 2021
Food and Drug Administration (2016) Avelumab drug approval package. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761049orig1s000multidiscipliner.pdf. Accessed 17 Feb 2021
Odogwu L, Mathieu L, Blumenthal G, Larkins E, Goldberg KB, Griffin N, Bijwaard K, Lee EY, Philip R, Jiang X, Rodriguez L, McKee AE, Keegan P, Pazdur R (2018) Fda approval summary: Dabrafenib and trametinib for the treatment of metastatic non-small cell lung cancers harboring braf v600e mutations. Oncologist 23(6):740–745. https://doi.org/10.1634/theoncologist.2017-0642
Food and Drug Administration (2019) Capmatinib drug approval package. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213591Orig1s000MultidisciplineR.pdf. Accessed 17 Feb 2021
Food and Drug Administration (2017) Axicabtagene ciloleuce drug approval package
Food and Drug Administration (2001) E 10 choice of control group and related issues in clinical trials. https://www.fda.gov/media/71349/download. Accessed 17 Feb 2021
Davies J, Martinec M, Delmar P, Coudert M, Bordogna W, Golding S, Martina R, Crane G (2018) Comparative effectiveness from a single-arm trial and real-world data: alectinib versus ceritinib. J Comp Effect Res 7(9):855–865. https://doi.org/10.2217/cer-2018-0032
Bartlett CH, Mardekian J, Cotter MJ, Huang X, Zhang Z, Parrinello CM, Bourla AB (2020) Concordance of real-world versus conventional progression-free survival from a phase 3 trial of endocrine therapy as first-line treatment for metastatic breast cancer. PLoS ONE 15(4):1–16. https://doi.org/10.1371/journal.pone.0227256
Carrigan G, Whipple S, Capra WB, Taylor MD, Brown JS, Lu M, Arnieri B, Copping R, Rothman KJ (2020) Using electronic health records to derive control arms for early phase single-arm lung cancer trials: proof-of-concept in randomized controlled trials. Clin Pharmacol Ther 107(2):369–377. https://doi.org/10.1002/cpt.1586
Ford I, Norrie J (2016) Pragmatic trials. N Engl J Med 375(5):454–463. https://doi.org/10.1056/NEJMra1510059
Zhu M, Sridhar S, Hollingsworth R, Chit A, Kimball T, Murmello K, Greenberg M, Gurunathan S, Chen J (2020) Hybrid clinical trials to generate real-world evidence: design considerations from a sponsor’s perspective. Contemp Clin Trials 94:105856. https://doi.org/10.1016/j.cct.2019.105856
Viele K, Berry S, Neuenschwander B, Amzal B, Chen F, Enas N, Hobbs B, Ibrahim JG, Kinnersley N, Lindborg S, Micallef S, Roychoudhury S, Thompson L (2014) Use of historical control data for assessing treatment effects in clinical trials. Pharm Stat 13(1):41–54
Wadsworth I, Hampson LV, Jaki T (2018) Extrapolation of efficacy and other data to support the development of new medicines for children: a systematic review of methods. Stat Methods Med Res 27(2):398–413. https://doi.org/10.1177/0962280216631359
Pocock SJ (1976) The combination of randomized and historical controls in clinical trials. J Chronic Dis 29(3):175–188
Stuart EA, Rubin DB (2008) Matching with multiple control groups with adjustment for group differences. J Educ Behav Stat 33(3):279–306. https://doi.org/10.3102/1076998607306078
Chen MH, Ibrahim JG (2000) Power prior distributions for regression models. Stat Sci 15(1):46–60. https://doi.org/10.1214/ss/1009212673
Duan Y, Ye K, Smith EP (2006) Evaluating water quality using power priors to incorporate historical information. Environmetrics 17(1):95–106. https://doi.org/10.1002/env.752
Neuenschwander B, Branson M, Spiegelhalter DJ (2009) A note on the power prior. Stat Med 28(28):3562–3566. https://doi.org/10.1002/sim.3722
Neuenschwander B, Capkun-Niggli G, Branson M, Spiegelhalter DJ (2010) Summarizing historical information on controls in clinical trials. Clin Trials 7(1):5–18. https://doi.org/10.1177/1740774509356002
Hobbs BP, Carlin BP, Mandrekar SJ, Sargent DJ (2011) Hierarchical commensurate and power prior models for adaptive incorporation of historical information in clinical trials. Biometrics 67(3):1047–1056. https://doi.org/10.1111/j.1541-0420.2011.01564.x
Gamalo-Siebers M, Savic J, Basu C, Zhao X, Gopalakrishnan M, Gao A, Song G, Baygani S, Thompson L, Xia HA, Price K, Tiwari R, Carlin BP (2017) Statistical modeling for Bayesian extrapolation of adult clinical trial information in pediatric drug evaluation. Pharm Stat 16(4):232–249
Dejardin D, Delmar P, Warne C, Patel K, van Rosmalen J, Lesaffre E (2018) Use of a historical control group in a noninferiority trial assessing a new antibacterial treatment: a case study and discussion of practical implementation aspects. Pharm Stat 17(2):169–181. https://doi.org/10.1002/pst.1843
van Rosmalen J, Dejardin D, van Norden Y, Löwenberg B, Lesaffre E (2018) Including historical data in the analysis of clinical trials: Is it worth the effort? Stat Methods Med Res 27(10):3167–3182
Rubin D (1974) Estimating causal effects of treatments in randomized and nonrandomized studies. J Educ Psychol 66(5):688–701
Rubin DB (1980) Randomization analysis of experimental data: the fisher randomization test comment. J Am Stat Assoc 75(371):591–593, http://www.jstor.org/stable/2287653
Rubin DB (1986) Statistics and causal inference: comment: which ifs have causal answers. J Am Stat Assoc 81(396):961–962, http://www.jstor.org/stable/2289065
Imbens GW, Rubin DB (2015) Causal Inference for Statistics, Social, and Biomedical Sciences: An Introduction. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9781139025751
Rosenbaum PR, Rubin DB (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70(1):41–55
Stuart EA (2010) Matching methods for causal inference: a review and a look forward. Stat Sci 25(1):1–21. https://doi.org/10.1214/09-STS313
Rubin DB (1973) Matching to remove bias in observational studies. Biometrics 29(1):159–183
Rosenbaum PR (2002) Observational Studies, 2nd edn. Springer, New York
Rubin DB (1987) Multiple Imputation for Nonresponse in Surveys. Wiley, New York
Ibrahim JG, Chen MH (2000) Power prior distributions for regression models. Statist Sci 15(1):46–60. https://doi.org/10.1214/ss/1009212673
Franklin JM, Schneeweiss S, Polinski JM, Rassen JA (2014) Plasmode simulation for the evaluation of pharmacoepidemiologic methods in complex healthcare databases. Comput Stat Data Anal 72:219–226
Fialkowski AC (2018) SimMultiCorrData: simulation of correlated data with multiple variable types. https://CRAN.R-project.org/package=SimMultiCorrData, r package version 0.2.2
Burcu M, Dreyer NA, Franklin JM, Blum MD, Critchlow CW, Perfetto EM, Zhou W (2020) Real-world evidence to support regulatory decision-making for medicines: considerations for external control arms. Pharmacoepidemiol Drug Saf 29(10):1228–1235. https://doi.org/10.1002/pds.4975
Psioda MA, Ibrahim JG (2018) Bayesian clinical trial design using historical data that inform the treatment effect. Biostatistics 20(3):400–415
Kopp-Schneider A, Calderazzo S, Wiesenfarth M (2020) Power gains by using external information in clinical trials are typically not possible when requiring strict type i error control. Biom J 62(2):361–374
Normington J, Zhu J, Mattiello F, Sarkar S, Carlin B (2020) An efficient Bayesian platform trial design for borrowing adaptively from historical control data in lymphoma. Contemp Clin Trials 89:105890. https://doi.org/10.1016/j.cct.2019.105890
Robins JM, Rotnitzky A, Zhao LP (1994) Estimation of regression coefficients when some regressors are not always observed. J Am Stat Assoc 89(427):846–866
Cole SR, Hernán MA (2008) Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168(6):656–664
Lee BK, Lessler J, Stuart EA (2011) Weight trimming and propensity score weighting. PLoS ONE 6:1–6. https://doi.org/10.1371/journal.pone.0018174
Austin PC, Stuart EA (2015) Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 34(28):3661–3679. https://doi.org/10.1002/sim.6607
Lin J, Gamalo-Siebers M, Tiwari R (2019) Propensity-score-based priors for Bayesian augmented control design. Pharm Stat 18(2):223–238. https://doi.org/10.1002/pst.1918
Wang C, Li H, Chen WC, Lu N, Tiwari R, Xu Y, Yue LQ (2019) Propensity score-integrated power prior approach for incorporating real-world evidence in single-arm clinical studies. J Biopharm Stat 29(5):731–748. https://doi.org/10.1080/10543406.2019.1657133
Wang C, Lu N, Chen WC, Li H, Tiwari R, Xu Y, Yue LQ (2020) Propensity score-integrated composite likelihood approach for incorporating real-world evidence in single-arm clinical studies. J Biopharm Stat 30(3):495–507. https://doi.org/10.1080/10543406.2019.1684309
Liu M, Bunn V, Hupf B, Lin J, Lin J (2021) Propensity-score-based meta-analytic predictive prior for incorporating real-world and historical data. Stat Med. https://doi.org/10.1002/sim.9095
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shan, M., Faries, D., Dang, A. et al. A Simulation-Based Evaluation of Statistical Methods for Hybrid Real-World Control Arms in Clinical Trials. Stat Biosci 14, 259–284 (2022). https://doi.org/10.1007/s12561-022-09334-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12561-022-09334-w