Abstract
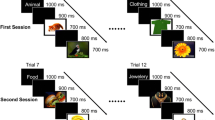
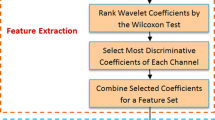
The problem of object recognition is solved in the brain using different strategies. These strategies are to some extent known to neuroscientists, but researches on this issue are still in progress to understand more accurately the computational, anatomical, and physiological aspects of this fast and accurate capability of the brain. In this paper, we presented a method, based on extracting nonlinearity of signals such as L-Z complexity, fractal dimension, Lyapunov exponents, Hurst exponents, and entropy, to classify single trials into their related semantic category groups with a linear SVM classifier. Furthermore, we proposed to combine nonlinear features mentioned above with wavelet coefficients to improve the classification accuracy. EEG signals were recorded from human subjects according to 10–20 system while performing a “go/no go” object-categorization task. Combining nonlinear features with wavelet coefficients led to a significant enhancement in classification accuracy (73%) relative to wavelet coefficients alone (54%). Feature-selection results showed that a significantly larger proportion of final selected features include nonlinear features (44%) relative to the first ratio of them (14%) to whole features. This ratio enhancement demonstrates the essential role of nonlinear features in the obtained classification accuracy. In addition, C3 channel and Katz fractal dimension were introduced as the most informative channel and the best nonlinear feature, respectively.







Similar content being viewed by others
References
DiCarlo JJ, Zoccolan D, Rust NC. How does the brain solve visual object recognition? Neuron. 2012;73(3):415–34.
Kourtzi Z, Connor CE. Neural representations for object perception: structure, category, and adaptive coding. Annu Rev Neurosci. 2011;34:45–67.
Seger CA, Miller EK. Category learning in the brain. Annu Rev Neurosci. 2010;33:203–19.
Rosch E. Cognitive representations of semantic categories. J Exp Psychol Gen. 1975;104(3):192.
Fabre-Thorpe M, Richard G, Thorpe SJ. Rapid categorization of natural images by rhesus monkeys. Neuroreport. 1998;9(2):303–8.
Freedman DJ, et al. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291(5502):312–6.
Macé MJ-M, et al. Rapid categorization of natural scenes in monkeys: target predictability and processing speed. Neuroreport. 2005;16(4):349–54.
Reddy L, Kanwisher N. Category selectivity in the ventral visual pathway confers robustness to clutter and diverted attention. Curr Biol. 2007;17(23):2067–72.
Mormann F, et al. A category-specific response to animals in the right human amygdala. Nat Neurosci. 2011;14(10):1247–9.
Cauchoix M, et al. Fast ventral stream neural activity enables rapid visual categorization. NeuroImage. 2016;125:280–90.
Goddard E, et al. Representational dynamics of object recognition: feedforward and feedback information flows. NeuroImage. 2016;128:385–97.
Connor CE, Brincat SL, Pasupathy A. Transformation of shape information in the ventral pathway. Curr Opin Neurobiol. 2007;17(2):140–7.
Lowe DG. Object recognition from local scale-invariant features. In Computer vision, 1999. The proceedings of the seventh IEEE international conference on. 1999: Ieee.
Fukushima K. Neocognitron: a self-organizing neural network model for a mechanism of pattern recognition unaffected by shift in position. Biol Cybern. 1980;36(4):193–202.
Riesenhuber M, Poggio T. Hierarchical models of object recognition in cortex. Nat Neurosci. 1999;2(11):1019–25.
Pinto N, et al. A high-throughput screening approach to discovering good forms of biologically inspired visual representation. PLoS Comput Biol. 2009;5(11):e1000579.
Freedman DJ, et al. Visual categorization and the primate prefrontal cortex: neurophysiology and behavior. J Neurophysiol. 2002;88(2):929–41.
Freedman DJ, et al. A comparison of primate prefrontal and inferior temporal cortices during visual categorization. J Neurosci. 2003;23(12):5235–46.
Bar M, et al. Top-down facilitation of visual recognition. Proc Natl Acad Sci U S A. 2006;103(2):449–54.
McKee JL, et al. Task dependence of visual and category representations in prefrontal and inferior temporal cortices. J Neurosci. 2014;34(48):16065–75.
Baldassi C, et al. Shape similarity, better than semantic membership, accounts for the structure of visual object representations in a population of monkey inferotemporal neurons. PLoS Comput Biol. 2013;9(8):e1003167.
Rodríguez-Bermúdez G, García-Laencina PJ. Automatic and adaptive classification of electroencephalographic signals for brain computer interfaces. J Med Syst. 2012;36(1):51–63.
Ramoser H, Muller-Gerking J, Pfurtscheller G. Optimal spatial filtering of single trial EEG during imagined hand movement. IEEE Trans Rehab Eng. 2000;8(4):441–6.
Sun H, et al. On-line EEG classification for brain-computer interface based on CSP and SVM. In Image and Signal Processing (CISP), 2010 3rd International Congress on. 2010: IEEE.
Krusienski DJ, McFarland DJ, Wolpaw JR. An evaluation of autoregressive spectral estimation model order for brain-computer interface applications. In Engineering in Medicine and Biology Society, 2006. EMBS’06. 28th Annual International Conference of the IEEE. 2006: IEEE.
Zhang Y, et al. Classification of EEG signals based on autoregressive model and wavelet packet decomposition. Neural Process Lett. 2016:1–14.
Taghizadeh-Sarabi M, Daliri MR, Niksirat KS. Decoding objects of basic categories from electroencephalographic signals using wavelet transform and support vector machines. Brain Topogr. 2015;28(1):33–46.
Jafakesh S, Jahromy FZ, Daliri MR. Decoding of object categories from brain signals using cross frequency coupling methods. Biomed Signal Process Control. 2016;27:60–7.
Jalili M. Multivariate synchronization analysis of brain electroencephalography signals: a review of two methods. Cogn Comput. 2015;7(1):3–10.
Orozco-Arroyave JR, et al. Nonlinear dynamics for hypernasality detection in Spanish vowels and words. Cogn Comput. 2013;5(4):448–57.
Arias-Londono JD, et al. Automatic detection of pathological voices using complexity measures, noise parameters, and mel-cepstral coefficients. IEEE Trans Biomed Eng. 2011;58(2):370–9.
Acharya UR, et al. A novel depression diagnosis index using nonlinear features in EEG signals. Eur Neurol. 2015;74(1–2):79–83.
Acharya R, et al. Non-linear analysis of EEG signals at various sleep stages. Comput Methods Prog Biomed. 2005;80(1):37–45.
Spratling M. A hierarchical predictive coding model of object recognition in natural images. Cogn Comput. 2016:1–17.
Greenspan H, van Ginneken B, Summers RM. Guest editorial deep learning in medical imaging: overview and future promise of an exciting new technique. IEEE Trans Med Imaging. 2016;35(5):1153–9.
Ghesu FC, et al. Marginal space deep learning: efficient architecture for volumetric image parsing. IEEE Trans Med Imaging. 2016;35(5):1217–28.
Bar Y, et al. Deep learning with non-medical training used for chest pathology identification. In SPIE Medical Imaging. 2015: International Society for Optics and Photonics.
Liskowski P, Krawiec K. Segmenting retinal blood vessels with<? Pub _newline?> deep neural networks. IEEE Trans Med Imaging. 2016;35(11):2369–80.
Roth HR, et al. Improving computer-aided detection using Convolutional neural networks and random view aggregation. IEEE Trans Med Imaging. 2016;35(5):1170–81.
Manor R, Geva AB. Convolutional neural network for multi-category rapid serial visual presentation bci. Front Comput Neurosci. 2015;9
Manor R, Mishali L, Geva AB. Multimodal neural network for rapid serial visual presentation brain computer interface. Front Comput Neurosci. 2016;10
Lin Y-P, et al. Support vector machine for EEG signal classification during listening to emotional music. In Multimedia Signal Processing, 2008 I.E. 10th Workshop on. 2008: IEEE.
Zhang Y, et al. Comparison of classification methods on EEG signals based on wavelet packet decomposition. Neural Comput & Applic. 2015;26(5):1217–25.
Tanveer M. Robust and sparse linear programming twin support vector machines. Cogn Comput. 2015;7(1):137–49.
Sanei S, Chambers JA. EEG signal processing. John Wiley & Sons; 2013.
Stam CJ. Nonlinear dynamical analysis of EEG and MEG: review of an emerging field. Clin Neurophysiol. 2005;116(10):2266–301.
Galka A. Topics in nonlinear time series analysis: with implications for EEG analysis. Vol. 14, World Scientific. 2000.
Cao L. Practical method for determining the minimum embedding dimension of a scalar time series. Physica D: Nonlinear Phenomena. 1997;110(1):43–50.
Lempel A, Ziv J. On the complexity of finite sequences. IEEE Trans Inf Theory. 1976;22(1):75–81.
Aboy M, et al. Interpretation of the Lempel-Ziv complexity measure in the context of biomedical signal analysis. IEEE Trans Biomed Eng. 2006;53(11):2282–8.
Higuchi T. Approach to an irregular time series on the basis of the fractal theory. Physica D: Nonlinear Phenomena. 1988;31(2):277–83.
Katz MJ. Fractals and the analysis of waveforms. Comput Biol Med. 1988;18(3):145–56.
Lai D, Chen G. Statistical analysis of Lyapunov exponents from time series: a Jacobian approach. Math Comput Model. 1998;27(7):1–9.
Sano M, Sawada Y. Measurement of the Lyapunov spectrum from a chaotic time series. Phys Rev Lett. 1985;55(10):1082.
Güler NF, Übeyli ED, Güler I. Recurrent neural networks employing Lyapunov exponents for EEG signals classification. Expert Syst Appl. 2005;29(3):506–14.
Balli T, Palaniappan R. Classification of biological signals using linear and nonlinear features. Physiol Meas. 2010;31(7):903.
Kaplan I. Estimating the Hurst exponent. 2003. Available from: www.bearcave.com/misl/misl tech/wavelets/hurst/index.
Bruhn J, Röpcke H, Hoeft A. Approximate entropy as an electroencephalographic measure of anesthetic drug effect during desflurane anesthesia. J Am Soc Anesthesiol. 2000;92(3):715–26.
Pincus SM, Huang W-M. Approximate entropy: statistical properties and applications. Commun Stat-Theory Methods. 1992;21(11):3061–77.
Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Phys Heart Circ Phys. 2000;278(6):H2039–49.
Guo L, et al. Classification of EEG signals using relative wavelet energy and artificial neural networks. In Proceedings of the first ACM/SIGEVO Summit on Genetic and Evolutionary Computation. 2009: ACM.
Chang C-C, Lin C-J. LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol (TIST). 2011;2(3):27.
Gallese V, et al. Action recognition in the premotor cortex. Brain. 1996;119(2):593–609.
Rizzolatti G, et al. Premotor cortex and the recognition of motor actions. Cogn Brain Res. 1996;3(2):131–41.
Acknowledgments
This work was financially supported by the Iran Neural Technology Research Center, Iran University of Science and Technology, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Torabi, A., Zareayan Jahromy, F. & Daliri, M.R. Semantic Category-Based Classification Using Nonlinear Features and Wavelet Coefficients of Brain Signals. Cogn Comput 9, 702–711 (2017). https://doi.org/10.1007/s12559-017-9487-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12559-017-9487-z