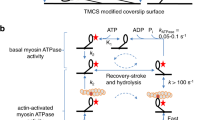
Abstract
Our knowledge in the field of cardiac muscle and associated cardiomyopathies has been evolving incrementally over the past 60 years and all was possible due to the parallel progress in techniques and methods allowing to take a fresh glimpse at an old problem. Here, we describe an exciting tool used to examine the various states of the human cardiac myosin at the single molecule level. By imaging single Alexa647-ATP binding to permeabilised cardiomyocytes using total internal reflection fluorescence (TIRF) microscopy, we are able to acquire large populations of events in a short timeframe (~ 5000 sites in ~ 10 min) and measure each binding event with high spatio-temporal resolution. The applied algorithm decomposes the point pattern of single molecule binding events into individually distinct binding sites that enables us to recover kinetic parameters, such as bound or free time per site. This single molecule binding approach is a useful tool used to examine muscle contractility. Of particular importance is its application to probing the dynamic lifetimes and proportion of myosins in the super-relaxed state in human cardiomyopathies.




Similar content being viewed by others
References
Alamo L, Ware JS, Pinto A et al (2017) Effects of myosin variants on interacting-heads motif explain distinct hypertrophic and dilated cardiomyopathy phenotypes. eLife 6:e24634
Anderson RL, Trivedi DV, Sarkar SS et al (2018) Deciphering the super relaxed state of human beta-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibres. Proc Natl Acad Sci U S A 115:E8143–E8152
Axelrod D (2001) Total internal reflection fluorescence microscopy in cell biology. Traffic 2:764–774
Carrier L, Hengstenberg C, Beckmann JS et al (1993) Mapping of a novel gene for familial hypertrophic cardiomyopathy to chromosome 11. Nature Genetics. 4:311–313
Carrier L, Mearini G, Stathopoulou K, Cuello F (2015) Cardiac myosin-binding protein C (MYBPC3) in cardiac pathophysiology. Gene. 573(2):188–197
Cooke R (1981) Stress does not alter the conformation of a domain of the myosin cross-bridge in rigor muscle fibres. Nature 294:570–571. https://doi.org/10.1038/294570a0
Dufresne E and Blair D (2005) The Matlab particle tracking code repository, http://site.physics.georgetown.edu/matlab/
Ebashi S (1963) Third component participating in the superprecipitation of ‘Natural actomyosin’. Nature 200:2010. https://doi.org/10.1038/2001010a0
Ferenczi MA, Homsher E, Simmons RM et al (1978) Reaction mechanism of the magnesium ion-dependent adenosine triphosphatase of frog muscle myosin and subfragment 1. Biochem J 171:165–175
Fish KN (2009) Total internal reflection fluorescence (TIRF) microscopy. Curr Protoc Cytom. Chapter 12
Hooijman P, Stewart MA, Cooke R (2011) A new state of cardiac myosin with very slow ATP turnover: a potential cardioprotective mechanism in the heart. Biophys J 100:1969–1976
Huxley HE, Hanson J (1954) Changes in the cross-striations of muscle during contractions and stretch and their structural interpretation. Nature 173:973–976
Huxley AF, Niedergerke R (1954) Structural changes in muscle during contraction. Nature 173:971–973
Ishiwata S, Funatsu T, Fujita H (1998) Contractile properties of thin (actin) filament-reconstituted muscle fibres. In: Pollack GH (ed) Sugi H. Mechanisms of work production and work absorption in muscle, Springer Science & Business Media, pp 319–329
Li A, Nelson SR, Rahmanseresht S, Braet S, Cornachione AS, Previs SB, O’Leary TS, McNamara JW, Rassier DE, Sadayappan S, Previs MJ, Warshaw DM (2019) Skeletal MyBP-C isoforms tune the molecular contractility of divergent skeletal muscle systems. Proc Natl Acad Sci 116(43):21882–21892
Lin BL, Li A, Mun JY, Previs MJ, Previs SB, Campbell SG, dos Remedios CG, Tombe PdP, Craig R, Warshaw DM, Sadayappan S. (2018) Skeletal myosin binding protein-C isoforms regulate thin filament activity in a Ca2+-dependent manner. Scientific Reports. 8:2604
Mamidi R, Li J, Doh CY et al (2018) Impact of the myosin modulator mavacamten on force generation and cross-bridge behavior in a murine model of hypercontractility. J Am Heart Assoc 7(17):e009627. https://doi.org/10.1161/JAHA.118.009627
McGrath PA, dos Remedios CG (1974) The dependence of rigor tension on sarcomere length in vertebrate muscle. Experientia 30:1036–1038
McNamara JW, Li A (2015) The role of super-relaxed myosin in skeletal and cardiac muscle. Biophys Rev 7:5–15
Morkel C, Pandzic E, Su Y-Y et al (2019) Direct observation of myosin cross-bridge heads while they hydrolyze ATP in cardiomyocytes from healthy donors and end-stage human heart failure. 43rd Ann. Conf Proc Aust Soc Biophys
Myberg KH, Franks-Skiba K, Cooke R (1995) Nucleotide turnover rate measured in fully relaxed rabbit skeletal muscle myofibrils. J Gen Physiol 106:957–973
Nelson SR, Li A, Beck-Previs S, Kennedy GG, Warshaw DM (2020) Imaging ATP consumption in resting skeletal muscle: one molecule at a time. bioRxiv 2020.05.27.119065. https://doi.org/10.1101/2020.05.27.119065
Offer G, Starr R (1971) Polypeptide chains of intermediate molecular weight in myosin preparations. FEBS Lett 15:40–44. https://doi.org/10.1016/0014-5793(71)80075-3
Offer G, Moos C, Starr R (1973) A new protein of the thick filaments of vertebrate skeletal myofibrils: extraction, purification and characterization. J Mol Biol 74:653–662
Perry SV (2008) Background to the discovery of troponin and Setsuro Ebashi’s contribution to our knowledge of the mechanism of relaxation in striated muscle. Biochem Biophys Res Commun 369:43–48. https://doi.org/10.1016/j.bbrc.2007.11.185
Ponnam S, Sevrieva I, Sun YB, Irving M, Kampourakis T (2019) Site-specific phosphorylation of myosin binding protein-C coordinates thin and thick filament activation in cardiac muscle. PNAS 116(31):15485–15494
Rassier DE (2008) Pre-power stroke cross bridge contribute to force during stretch of skeletal muscle myofibrils. Proc R Soc B 275:2577–2586
Stewart MA, Franks-Skiba K, Chen S et al (2010) Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc Natl Acad Sci U S A 107:430–435
Tonino P, Kiss B, Gohlke J, Smith JE, Granzier H (2019) Fine mapping titin's C-zone: Matching cardiac myosin-binding protein C stripes with titin's super-repeats. J Mol Cell Cardiol 133:47–56
Tsaturyan AK, Bershitsky SY, Burns R, He Z-H, Ferenczi MA (1999) J Physiol 520(3):681–696
Walcott S, Docken S, Harris SP (2015) Effects of cardiac myosin binding protein-C on actin motility are explained with a drag-activation-competition model. Biophys J 108(1):10–13. https://doi.org/10.1016/j.bpj.2014.11.1852
Weber K, Osborn M (1969) The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem 244:4406–4412
Weber FE, Vaughan KT, Reinach FC, Fischman (1993) Complete Sequence of Human Fast-Type and Slow-Type Muscle Myosin-Binding-Protein C (MyBP-C). Differential Expression, Conserved Domain Structure and Chromosome Assignment. Eur J Biochem. 216(2):661–669
White DCS (1970) Rigor contraction and the effect of various phosphate compounds on glycerinated insect flight and vertebrate muscle. J Physiol 208:583–605
Wilson C, Naber N, Pate E, Cooke R (2014) The myosin inhibitor blebbistatin stabilizes the super-relaxed state in skeletal muscle. Biophys J 107:1637–1646
Xu S, White HD, Yu LC (2009) Stabilization of helical order in the thick filaments by blebbistatin: further evidence of coexisting multiple conformations of myosin. Biophys J 96:3673–3681
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pandzic, E., Morkel, C.A., Li, A. et al. Nanomolar ATP binding to single myosin cross-bridges in rigor: a molecular approach to studying myosin ATP kinetics using single human cardiomyocytes. Biophys Rev 12, 1031–1040 (2020). https://doi.org/10.1007/s12551-020-00716-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-020-00716-2