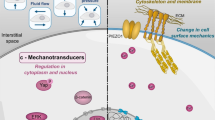
Abstract
Organoids are in vitro 3D self-organizing tissues that mimic embryogenesis. Organoid research is advancing at a tremendous pace, since it offers great opportunities for disease modeling, drug development and screening, personalized medicine, as well as understanding organogenesis. Mechanobiology of organoids is an unexplored area, which can shed light to several unexplained aspects of self-organization behavior in organogenesis. It is becoming evident that collective cell behavior is distinctly different from individual cells’ conduct against certain stimulants. Inherently consisting of higher number of degrees of freedom for cell motility and more complex cell-to-cell and cell-to-extracellular matrix behavior, understanding mechanotransduction in organoids is even more challenging compared with cell communities in 2D culture conditions. Yet, deciphering mechanobiology of organoids can help us understand effects of mechanical cues in health and disease, and translate findings of basic research toward clinical diagnosis and therapy.
Similar content being viewed by others
References
Akhmanova M, Osidak E, Domogatsky S, Rodin S, Domogatskaya A (2015) Physical, spatial, and molecular aspects of extracellular matrix of in vivo niches and artificial scaffolds relevant to stem cells research. Stem Cells Int 35
Alberts B, Johnson A, Lewis J, Raff M, Roberts K and Walter P (2002) The extracellular matrix of animals. In: Molecular biology of the cell. 4th edition. New York Garland Science. https://www.ncbi.nlm.nih.gov/books/NBK26810/. Accessed 24 June 2019
Allende ML et al (2018) Cerebral organoids derived from Sandhoff disease-induced pluripotent stem cells exhibit impaired neurodifferentiation. J Lipid Res 59:550–563
Ambrosini A et al (2019) Mechanical function of the nucleus in force generation during epithelial morphogenesis. Dev Cell 50:1–15
Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ (2005) Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science 307:423–426
Ardhanareeswaran K, Mariani J, Coppola G, Abyzov A, Vaccarino FM (2017) Human induced pluripotent stem cells for modelling neurodevelopmental disorders. Nat Rev Neurol 13:265–278
Ashby WR (1962) Principles of the self-organizing system. In: Von Foerster H, Zopf GW (eds) Principles of self-organization: transactions of the University of Illinois Symposium. Pergamon Press, London, UK, pp 255–278
Baker BM, Chen CS (2012) Deconstructing the third dimension–how 3D culture microenvironments alter cellular cues. J Cell Sci 125:3015–3024
Baumgarten CM (2013) Origin of mechanotransduction: stretch-activated ion channels. In: Madame curie bioscience database [Internet]. Austin (TX), Landes Bioscience, pp 2000–2013 Available from: https://www.ncbi.nlm.nih.gov/books/NBK6374/. Accessed 27 June 2019
Beningo KA, Dembo M, Kaverina I, Small JV, Wang Y-l (2001) Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol 153:881–888
Berger E et al (2018) Millifluidic culture improves human midbrain organoid vitality and differentiation. Lab Chip 18:3172–3183
Bhat SP (2001) The ocular lens epithelium. Biosci Rep 21:537–563. https://doi.org/10.1023/A:1017952128502
Buske P, Przybilla J, Loeffler M, Sachs N, Sato T, Clevers H, Galle J (2012) On the biomechanics of stem cell niche formation in the gut–modelling growing organoids. FEBS J 279:3475–3487
Cerruti B et al (2013) Polarity, cell division, and out-of-equilibrium dynamics control the growth of epithelial structures. J Cell Biol 203(2):359–372
Chan CJ, Heisenberg C-P, Hiiragi T (2017) Coordination of morphogenesis and cell-fate specification in development. Curr Biol 27:R1024–R1035
Classen AK, Anderson KI, Marois E, Eaton S (2005) Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell 9:805–817. https://doi.org/10.1016/j.devcel.2005.10.016
Coste et al (2012) Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483:176–181
Cukierman E, Pankov R, Stevens DR, Yamada KM (2001) Taking cell-matrix adhesions to the third dimension. Science 294:1708–1712
Dahl-Jensen SB, Figueiredo-Larsen M, Grapin-Botton A, Sneppen K (2016) Short-range growth inhibitory signals from the epithelium can drive non-stereotypic branching in the pancreas. Phys Biol 13:016007
Drost J, Clevers H (2017) Translational applications of adult stem cell-derived organoids. Development 144:968–975
Dupont et al (2011) Role of YAP/TAZ in mechanotransduction. Nature 474:179–183
Dutta D, Heo I, Clevers H (2017) Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med 23:393–410
Duval K, Grover H, Han L-H, Mou Y, Pegoraro AF, Fredberg J, Chen Z (2017) Modeling physiological events in 2D vs. 3D cell culture. Physiology 32:266–277
Eiraku M, Adachi T, Sasai Y (2012) Relaxation-expansion model for self-driven retinal morphogenesis. Bioessays 34(1):17–25
Ellison D et al (2016) Cell-cell communication enhances the capacity of cell ensembles to sense shallow gradients during morphogenesis. Proceedings of the National Academy of Sciences of the United States of America (PNAS) 113(6):E679–88
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126:677–689
Eshghi S, Schaffer DV (2008) Engineering microenvironments to control stem cell fate and function. In: StemBook [Internet]. Ed. Lisa Girard Harvard Stem Cell Institute. https://doi.org/10.3824/stembook.1.5.1
Fang Y, Eglen RM (2017) Three-dimensional cell cultures in drug discovery and development. Slas discovery: Advancing Life Sciences R&D 22:456–472
Fiorotto R, Amenduni M, Mariotti V, Fabris L, Spirli C, Strazzabosco M (2018) Liver diseases in the dish: iPSC and organoids as a new approach to modeling liver diseases. Biochim Biophys Acta, Mol Basis Dis 1865(5):920–928. https://doi.org/10.1016/j.bbadis.2018.08.038
Fordham RP, Yui S, Hannan NRF, Soendergaard C, Madgwick A et al (2013) Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13:734–744
Frisch SM, Francis H (1994) Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124:619–626
Gähwiler B, Capogna M, Debanne D, McKinney R, Thompson S (1997) Organotypic slice cultures: a technique has come of age. Trends Neurosci 20:471–477
Gaitantzi H, Breitkopf-Heinlein K (2018) Spontaneous self-assembly of liver organoids from differentiated human cells: human liver organoids. In: Davies JA, Lawrence ML (eds) Organs and organoids. Elsevier, Part 2, Chapter 7 145–156
Gattazzo F, Urciuolo A, Bonaldo P (2014) Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta, Gen Subj 1840:2506–2519
Geiger B, Yamada KM (2011) Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol 3(5):a005033. https://doi.org/10.1101/cshperspect.a005033
Greggio C, De Franceschi F, Figueiredo-Larsen M, Gobaa S, Ranga A, Semb H, Lutolf M, Grapin-Botton A (2013) Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 140:4452–4462
Ho B, Pek N, Soh B-S (2018) Disease modeling using 3D organoids derived from human induced pluripotent stem cells. Int J Mol Sci 19:936
Hofer H, Carroll J, Neitz J, Neitz M, Williams DR (2005) Organization of the human trichromatic cone mosaic. J Neurosci 25:9669–9679. https://doi.org/10.1523/JNEUROSCI.2414-05.2005
Irie Y, Mizumoto H, Fujino S, Kajiwara T (2008) Development of articular cartilage grafts using organoid formation techniques. Transplant Proc 40(2):631–633
Jackson EL, Lu H (2016) Three-dimensional models for studying development and disease: moving on from organisms to organs-on-a-chip and organoids. Integr Biol 8:672–683
Jalbert I, Stapleton F, Papas E, Sweeney DF, Coroneo M (2003) In vivo confocal microscopy of the human cornea. Br J Ophthalmol 87:225–236. https://doi.org/10.1136/bjo.87.2.225
Jansen KA, Donato DM, Balcioglu HE, Schmidt T, Danen EH, Koenderink GH (2015) A guide to mechanobiology: where biology and physics meet. Biochim Biophys Acta, Mol Cell Res 1853(11B):3043–3052
Kapałczyńska M et al (2018) 2D and 3D cell cultures–a comparison of different types of cancer cell cultures. Arch Med Sci 14(4):910–919. https://doi.org/10.5114/aoms.2016.63743
Karolak A, Markov DA, McCawley LJ, Rejniak KA (2018) Towards personalized computational oncology: from spatial models of tumour spheroids, to organoids, to tissues. J R Soc Interface 15:20170703. https://doi.org/10.1098/rsif.2017.0703
Karzbrun E, Reiner O (2019) Brain organoids—a bottom-up approach for studying human neurodevelopment. Bioengineering 6(1):9. https://doi.org/10.3390/bioengineering6010009
Kim YK, Nam SA, Yang CW (2018) Applications of kidney organoids derived from human pluripotent stem cells. Korean J Intern Med 33(4):649–659
Kirby TJ, Lammerding J (2018) Emerging views of the nucleus as a cellular mechanosensor. Nat Cell Biol 20:373–381
Kohl P (2018) Cardiac stretch-activated channels and mechano-electric coupling. In: Zipes DP, Jalife J, Stevenson WG (eds) Cardiac electrophysiology: from cell to bedside (Seventh Edition). Elsevier Inc. Chapter 14 128–139
Lancaster MA, Knoblich JA (2014) Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345(6194):1247125. https://doi.org/10.1126/science.1247125
Lancaster MA et al (2013) Cerebral organoids model human brain development and microcephaly. Nature 501:373–379
Leipzig ND, Shoichet MS (2009) The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 30:6867–6878
Li Z, Lee H, Zhu C (2016) Molecular mechanisms of mechanotransduction in integrin-mediated cell-matrix adhesion. Exp Cell Res 349:85–94. https://doi.org/10.1016/j.yexcr.2016.10.001
Mammoto T, Mammoto A, Ingber DE (2013) Mechanobiology and developmental control. Annu Rev Cell Dev Biol 29:27–61
Mariani J et al (2015) FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162:375–390
Martinac B (2014) The ion channels to cytoskeleton connection as potential mechanism of mechanosensitivity. Biochim Biophys Acta 1838:682–691
Martino F, Perestrelo AR, Vinarský V, Pagliari S, Forte G (2018) Cellular mechanotransduction: from tension to function. Front Physiol 9:824
Milde F, Tauriello G, Haberkern H, Koumoutsakos P (2014) SEM++: a particle model of cellular growth, signaling and migration. Comput Particle Mech 1(2):211–227
Miroshnikova YA, Nava MM, Wickstroem SA (2017) Emerging roles of mechanical forces in chromatin regulation. J Cell Sci 130:2243–2250
Misra M, Audoly B, Kevrekidis IG, Shvartsman SY (2016) Shape transformations of epithelial shells. Biophys J 110(7):1670–1678. https://doi.org/10.1016/j.bpj.2016.03.009
Missirlis YF (2016) Mechanoepigenetics. Front Cell Dev Biol 4:113
Mizuno S, Takada E, Fukai N (2016) Spheroidal organoids reproduce characteristics of longitudinal depth zones in bovine articular cartilage. Cells Tissues Organs 202:382–392
Muncie JM, Weaver VM (2018) The physical and biochemical properties of the extracellular matrix regulate cell fate. Curr Top Dev Biol 130:1–37
Na S et al (2008) Rapid signal transduction in living cells is a unique feature of mechanotransduction. PNAS 105(18):6626–6631
Nakamura T, Sato T (2018) Advancing intestinal organoid technology toward regenerative medicine. Cell Mol Gastroenterol Hepatol 5:51–60
Park SE, Georgescu A, Huh D (2019) Organoids-on-a-chip. Science 364:960–965
Pertz O et al (2006) Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 440:1069–1072
Poli D, Magliaro C, Ahluwalia A (2019) Experimental and computational methods for the study of cerebral organoids: a review. Front Neurosci 13:162
Portrian D et al (2017) Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat Cell Biol 19:391–398
Qian X et al (2016) Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165:1238–1254
Qian X, Song H, Ming G-L (2019) Brain organoids: advances, applications and challenges. Development 146:dev166074. https://doi.org/10.1242/dev.166074
Qiu X, Müller U (2018) Mechanically gated ion channels in mammalian hair cells. Front Cell Neurosci 12:100
Rahmani S, Breyner NM, Su H-M, Verdu EF, Didar TF (2018) Intestinal organoids: a new paradigm for engineering intestinal epithelium in vitro. Biomaterials 194:195–214
Seo J et al (2017) Inhibition of p25/Cdk5 attenuates tauopathy in mouse and iPSC models of frontotemporal dementia. J Neurosci 37:9917–9924
Singh A et al (2018) Polarized microtubule dynamics directs cell mechanics and coordinates forces during epithelial morphogenesis. Nat Cell Biol 20:1126–1133
Stachowiak MR et al (2014) A mechanical-biochemical feedback loop regulates remodeling in the actin cytoskeleton. PNAS 111(49):17528–17533
Stathopoulos A, Iber D (2013) Studies of morphogens: keep calm and carry on. Development 140:4119–4124
Sugawara T, Sasaki K, Akutsu H (2018) Organoids recapitulate organs? Stem Cell Investig 5:3. https://doi.org/10.21037/sci.2018.01.02
Sunyer R, Trepat X (2017) Mechanobiology of collective cell systems. Biofisica 7:1–7 http://biofisica.info. Accessed 18 June 2019
Suzuki A et al (2016) How the kinetochore couples microtubule force and centromere stretch to move chromosomes. Nat Cell Biol 18:382–392
Swift J et al (2013) Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341(6149):1240104. https://doi.org/10.1126/science.1240104
Tajik A et al (2016) Transcription upregulation via force-induced direct stretching of chromatin. Nat Mater 15:1287–1296
Todhunter ME, Jee NY, Hughes AJ, Coyle MC, Cerchiari A, Farlow J, Garbe JC, LaBarge MA, Desai TA, Gartner ZJ (2015) Programmed synthesis of three-dimensional tissues. Nat Methods 12:975–981
Turner DA, Baillie-Johnson P, Martinez Arias A (2016) Organoids and the genetically encoded self-assembly of embryonic stem cells. BioEssays 38:181–191
Ungrin MD, Clarke G, Yin T, Niebrugge S, Nostro MC, Sarangi F, Wood G, Keller G, Zandstra PW (2012) Rational bioprocess design for human pluripotent stem cell expansion and endoderm differentiation based on cellular dynamics. Biotechnol Bioeng 109:853–866
Vyas D et al (2018) Self-assembled liver organoids recapitulate hepatobiliary organogenesis in vitro. Hepatology 67:750–761
Watt FM, Huck WT (2013) Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol 14(8):467–473
Xu H, Jiao Y, Qin S, Zhao W, Chu Q, Wu K (2018) Organoid technology in disease modelling, drug development, personalized treatment and regeneration medicine. Exp Hematol Oncol 7:30. https://doi.org/10.1186/s40164-018-0122-9
Yin X, Mead BE, Safaee H, Langer R, Karp JM, Levy O (2016) Engineering stem cell organoids. Cell Stem Cell 18:25–38
Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B (2003) Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci 116:4605–4613
Zijl S, Lomakin AJ (2019) The “nuclear physics” behind genetic and epigenetic control of cell fate. Exp Cell Res 376(2):236–239
Acknowledgments
The authors acknowledge COST Action 16122 (BIONECA) for financing Prof. Dr. Yannis F. Missirlis for an STSM to visit and collaborate with the rest of the authors at Ege University; COST Action 16217 (ENIUS) for designating him as a disseminator to “promote” the Action at 4th international Symposium on Nanoengineering for Mechanobiology (N4M), where some of the ideas presented in this review were discussed; and Republic of Turkey Ministry of Development [EGEMATAL;2010K120810] for financing Dr. Ece Bayir.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bayir, E., Sendemir, A. & Missirlis, Y.F. Mechanobiology of cells and cell systems, such as organoids. Biophys Rev 11, 721–728 (2019). https://doi.org/10.1007/s12551-019-00590-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-019-00590-7