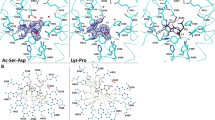
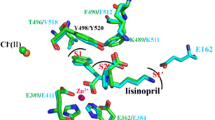
Abstract
Somatic angiotensin converting enzyme (sACE) is well-known for its role in blood pressure regulation and consequently, ACE inhibitors are widely prescribed for the treatment of hypertension. More than 60 years after the discovery of sACE, however, the molecular details of its substrate hydrolysis and inhibition are still poorly understood. Isothermal titration calorimetry, molecular dynamics simulations and fine epitope mapping suggest that substrate or inhibitor binding triggers a hinging motion between the two subdomains of each domain. Ligand binding to one domain further induces a conformational change in sACE to negatively affect the second domain’s function and can also cause dimerization between sACE molecules. This has been linked to an increase in sACE expression via intracellular signalling. Inhibitor-induced dimerization could thus decrease the efficacy of hypertension treatment. At present, the only structural information available for sACE are crystal structures of the truncated domains in the closed conformation due to the presence of ligands. These structures do not provide any information regarding the open active site conformation prior to ligand binding, the relative orientation of the two domains in full-length sACE, or the dimerization interface. To guarantee effective therapeutic intervention, further research is required to investigate the hinging, negative cooperativity and dimerization of sACE. This review describes our current understanding of these interactions and proposes how recent advances in cryo-electron microscopy could enable structural elucidation of their mechanisms.


Similar content being viewed by others
Abbreviations
- Aβ:
-
Amyloid beta
- ACE:
-
Angiotensin-converting enzyme
- ACE2:
-
Angiotensin-converting enzyme-2
- AcSDKP:
-
N-Acetyl serine-aspartate-lysine-proline
- AngI:
-
Angiotensin I
- EM:
-
Electron microscopy
- FRET:
-
Fluorescence resonance energy transfer
- ITC:
-
Isothermal titration calorimetry
- MD:
-
Molecular dynamics
- sACE:
-
Somatic ACE
- SAXS:
-
Small-angle X-ray scattering
- tACE:
-
Testis ACE
References
Abrie JA, Moolman WJA, Cozier GE et al (2018) Investigation into the mechanism of homo- and heterodimerization of angiotensin-converting enzyme. Mol Pharmacol 93:344–354
Acharya KR, Sturrock ED, Riordan JF, Ehlers MR (2003) Ace revisited: a new target for structure-based drug design. Nat Rev Drug Discov 2:891–902
Andujar-Sanchez M, Camara-Artigas A, Jara-Perez V (2004) A calorimetric study of the binding of lisinopril, enalaprilat and captopril to angiotensin-converting enzyme. Biophys Chem 111:183–189
Anthony CS, Corradi HR, Schwager SL et al (2010) The N domain of human angiotensin-I-converting enzyme: the role of N-glycosylation and the crystal structure in complex with an N domain-specific phosphinic inhibitor, RXP407. J Biol Chem 285:35685–35693
Araujo MC, Melo RL, Cesari MH et al (2000) Peptidase specificity characterization of C- and N-terminal catalytic sites of angiotensin I-converting enzyme. Biochemistry 39:8519–8525
Bai XC, McMullan G, Scheres SH (2015) How cryo-EM is revolutionizing structural biology. Trends Biochem Sci 40:49–57
Balyasnikova IV, Skirgello OE, Binevski PV et al (2007) Monoclonal antibodies 1G12 and 6A12 to the N-domain of human angiotensin-converting enzyme: fine epitope mapping and antibody-based detection of ACE inhibitors in human blood. J Proteome Res 6:1580–1594
Barauna VG, Campos LC, Miyakawa AA, Krieger JE (2011) ACE as a mechanosensor to shear stress influences the control of its own regulation via phosphorylation of cytoplasmic Ser(1270). PLoS One 6:e22803
Bernstein KE, Ong FS, Blackwell WB et al (2013) A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol Rev 65:1–46
Bicket DP (2002) Using ACE inhibitors appropriately. Am Fam Physician 66:461–468
Binevski PV, Sizova EA, Pozdnev VF, Kost OA (2003) Evidence for the negative cooperativity of the two active sites within bovine somatic angiotensin-converting enzyme. FEBS Lett 550:84–88
Ceska T, Chung CW, Cooke R et al (2019) Cryo-EM in drug discovery. Biochem Soc Trans 47:281–293
Chen HL, Lunsdorf H, Hecht HJ, Tsai H (2010) Porcine pulmonary angiotensin I-converting enzyme--biochemical characterization and spatial arrangement of the N- and C-domains by three-dimensional electron microscopic reconstruction. Micron 41:674–685
Cornell MJ, Williams TA, Lamango NS et al (1995) Cloning and expression of an evolutionary conserved single-domain angiotensin converting enzyme from Drosophila melanogaster. J Biol Chem 270:13613–13619
Corradi HR, Schwager SL, Nchinda AT et al (2006) Crystal structure of the N domain of human somatic angiotensin I-converting enzyme provides a structural basis for domain-specific inhibitor design. J Mol Biol 357:964–974
Danilov SM, Watermeyer JM, Balyasnikova IV et al (2007) Fine epitope mapping of monoclonal antibody 5F1 reveals anticatalytic activity toward the N domain of human angiotensin-converting enzyme. Biochemistry 46:9019–9031
Danilov SM, Gordon K, Nesterovitch AB et al (2011) An angiotensin I-converting enzyme mutation (Y465D) causes a dramatic increase in blood ACE via accelerated ACE shedding. PLoS One 6:e25952
Denti P, Sharp SK, Kroger WL et al (2014) Pharmacokinetic evaluation of lisinopril-tryptophan, a novel C-domain ACE inhibitor. Eur J Pharm Sci 56:113–119
Douglas RG, Sharma RK, Masuyer G et al (2014) Fragment-based design for the development of N-domain-selective angiotensin-1-converting enzyme inhibitors. Clin Sci 126:305–313
Ehlers MR, Gordon K, Schwager SL, Sturrock ED (2012) Shedding the load of hypertension: the proteolytic processing of angiotensin-converting enzyme. S Afr Med J 102:461–464
Ehlers MR, Abrie JA, Sturrock ED (2013) C domain-selective inhibition of angiotensin-converting enzyme. J Renin-Angiotensin-Aldosterone Syst 14:189–192
Erdos EG (1990) Angiotensin-I converting enzyme and the changes in our concepts through the years - Dahl,Lewis,K. Mem Lect Hypertens 16:363–370
Fang L, Geng M, Liu C et al (2019) Structural and molecular basis of angiotensin-converting enzyme by computational modeling: insights into the mechanisms of different inhibitors. PLoS One 14:e0215609
Fienberg S, Cozier GE, Acharya KR et al (2018) The design and development of a potent and selective novel diprolyl derivative that binds to the N-domain of angiotensin-I converting enzyme. J Med Chem 61:344–359
Fuchs S, Xiao HD, Hubert C et al (2008) Angiotensin-converting enzyme C-terminal catalytic domain is the main site of angiotensin I cleavage in vivo. Hypertension 51:267–274
Gordon K, Redelinghuys P, Schwager SL et al (2003) Deglycosylation, processing and crystallization of human testis angiotensin-converting enzyme. Biochem J 371:437–442
Gordon K, Balyasnikova IV, Nesterovitch AB et al (2010) Fine epitope mapping of monoclonal antibodies 9B9 and 3G8 to the N domain of angiotensin-converting enzyme (CD143) defines a region involved in regulating angiotensin-converting enzyme dimerization and shedding. Tissue Antigens 75:136–150
Hagaman JR, Moyer JS, Bachman ES et al (1998) Angiotensin-converting enzyme and male fertility. Proc Natl Acad Sci U S A 95:2552–2557
Herzik MA, Wu M, Lander GC (2019) High-resolution structure determination of sub-100 kDa complexes using conventional cryo-EM. Nat Commun 10:1032
Hines CS, Ray K, Schmidt JJ et al (2014) Allosteric inhibition of the neuropeptidase neurolysin. J Biol Chem 289:35605–35619
Jaspard E, Alhenc-Gelas F (1995) Catalytic properties of the two active sites of angiotensin I-converting enzyme on the cell surface. Biochem Biophys Res Commun 211:528–534
Jaspard E, Wei L, Alhenc-Gelas F (1993) Differences in the properties and enzymatic specificities of the two active sites of angiotensin I-converting enzyme (kininase II). Studies with bradykinin and other natural peptides. J Biol Chem 268:9496–9503
Kessler SP, Rowe TM, Gomos JB et al (2000) Physiological non-equivalence of the two isoforms of angiotensin-converting enzyme. J Biol Chem 275:26259–26264
Kohlstedt K, Gershome C, Friedrich M et al (2006) Angiotensin-converting enzyme (ACE) dimerization is the initial step in the ACE inhibitor-induced ACE signaling cascade in endothelial cells. Mol Pharmacol 69:1725–1732
Kost OA, Balyasnikova IV, Chemodanova EE et al (2003) Epitope-dependent blocking of the angiotensin-converting enzyme dimerization by monoclonal antibodies to the N-terminal domain of ACE: possible link of ACE dimerization and shedding from the cell surface. Biochemistry 42:6965–6976
Larmuth KM, Masuyer G, Douglas RG et al (2016) Kinetic and structural characterization of amyloid-beta peptide hydrolysis by human angiotensin-1-converting enzyme. FEBS J 283:1060–1076
Lubbe L, Sewell BT, Sturrock ED (2016) The influence of angiotensin converting enzyme mutations on the kinetics and dynamics of N-domain selective inhibition. FEBS J 283:3941–3961
Masuyer G, Douglas RG, Sturrock ED, Acharya KR (2015) Structural basis of Ac-SDKP hydrolysis by angiotensin-I converting enzyme. Sci Rep 5:13742
Michel B, Grima M, Nirina LB et al (2001) Inhibitory effect of reactive oxygen species on angiotensin I-converting enzyme (kininase II). Clin Exp Pharmacol Physiol 28:212–218
Murata K, Wolf M (2018) Cryo-electron microscopy for structural analysis of dynamic biological macromolecules. Biochim Biophys Acta Gen Subj 1862:324–334
Naperova IA, Balyasnikova IV, Schwartz DE et al (2008) Mapping of conformational mAb epitopes to the C domain of human angiotensin I-converting enzyme. J Proteome Res 7:3396–3411
Natesh R, Schwager SL, Sturrock ED, Acharya KR (2003) Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature 421:551–554
Ondetti MA, Rubin B, Cushman DW (1977) Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science (New York, NY) 196:441–444
O'Neill HG, Redelinghuys P, Schwager SL, Sturrock ED (2008) The role of glycosylation and domain interactions in the thermal stability of human angiotensin-converting enzyme. Biol Chem 389:1153–1161
Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM (2004) Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J 383:45–51
Rousseau A, Michaud A, Chauvet MT et al (1995) The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J Biol Chem 270:3656–3661
Shen XZ, Ong FS, Bernstein EA et al (2012) Nontraditional roles of angiotensin-converting enzyme. Hypertension (Dallas, Tex : 1979) 59:763–768
Skeggs LT Jr, Kahn JR, Shumway NP (1956) The preparation and function of the hypertensin-converting enzyme. J Exp Med 103:295–299
Skirgello OE, Binevski PV, Pozdnev VF, Kost OA (2005) Kinetic probes for inter-domain co-operation in human somatic angiotensin-converting enzyme. Biochem J 391:641–647
Skirgello OE, Balyasnikova IV, Binevski PV et al (2006) Inhibitory antibodies to human angiotensin-converting enzyme: fine epitope mapping and mechanism of action. Biochemistry 45:4831–4847
Soubrier F (1988) Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc Natl Acad Sci U S A 85:9386
Voronov S, Zueva N, Orlov V et al (2002) Temperature-induced selective death of the C-domain within angiotensin-converting enzyme molecule. FEBS Lett 522:77–82
Watermeyer JM, Sewell BT, Schwager SL et al (2006) Structure of testis ACE glycosylation mutants and evidence for conserved domain movement. Biochemistry 45:12654–12663
Watermeyer JM, Kroger WL, O'Neill HG et al (2008) Probing the basis of domain-dependent inhibition using novel ketone inhibitors of angiotensin-converting enzyme. Biochemistry 47:5942–5950
Watermeyer JM, Kroger WL, O'Neill HG et al (2010) Characterization of domain-selective inhibitor binding in angiotensin-converting enzyme using a novel derivative of lisinopril. Biochem J 428:67–74
Wei L (1991) The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J Biol Chem 266:9002
Wei L, Clauser E, Alhenc-Gelas F, Corvol P (1992) The two homologous domains of human angiotensin I-converting enzyme interact differently with competitive inhibitors. J Biol Chem 267:13398–13405
Woodman ZL, Schwager SL, Redelinghuys P et al (2005) The N domain of somatic angiotensin-converting enzyme negatively regulates ectodomain shedding and catalytic activity. Biochem J 389:739–744
Yates CJ, Masuyer G, Schwager SL et al (2014) Molecular and thermodynamic mechanisms of the chloride-dependent human angiotensin-I-converting enzyme (ACE). J Biol Chem 289:1798–1814
Yu XC, Sturrock ED, Wu Z et al (1997) Identification of N-linked glycosylation sites in human testis angiotensin-converting enzyme and expression of an active deglycosylated form. J Biol Chem 272:3511–3519
Funding
This work was supported by a UK Global Challenge Research Fund grant: START-Synchrotron Techniques for African Research and Technology (Science and Technology Facilities Council grant ref. ST/R002754/1) and the National Research Foundation of South Africa (Grant No. 111798).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Lizelle Lubbe declares that she has no conflict of interest. Edward D. Sturrock declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lubbe, L., Sturrock, E.D. Interacting cogs in the machinery of the renin angiotensin system. Biophys Rev 11, 583–589 (2019). https://doi.org/10.1007/s12551-019-00555-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-019-00555-w