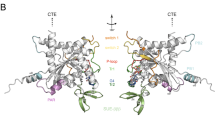
Abstract
Septins are able to polymerize into long apolar filaments and have long been considered to be a component of the cytoskeleton alongside intermediate filaments (which are also apolar in nature), microtubules and actin filaments (which are not). Their central guanosine triphosphate (GTP)-binding domain, which is essential for stabilizing the filament itself, is flanked by N- and C-terminal domains for which no direct structural information is yet available. In most cases, physiological filaments are built from a number of different septin monomers, and in the case of mammalian septins this is most commonly either three or four. Comprehending the structural basis for the spontaneous assembly of such filaments requires a deeper understanding of the interfaces between individual GTP-binding domains than is currently available. Nevertheless, in this review we will summarize the considerable progress which has been made over the course of the last 10 years. We will provide a brief description of each structure determined to date and comment on how it has added to the body of knowledge which is rapidly growing. Rather than simply repeat data which have already been described in the literature, as far as is possible we will try to take advantage of the full set of information now available (mostly derived from human septins) and draw the reader’s attention to some of the details of the structures themselves and the filaments they form which have not be commented on previously. An additional aim is to clarify some misconceptions.












Similar content being viewed by others
References
Angelis D, Karasmanis EP, Bai X, Spiliotis ET (2014) In silico docking of forchlorfenuron (FCF) to septins suggests that FCF interferes with GTP binding. PLoS One 9(5):e96390. https://doi.org/10.1371/journal.pone.0096390
Bai X, Bowen JR, Knox TK, Zhou K, Pendziwiat M, Kuhlenbäumer G, Sindelar CV, Spiliotis ET (2013) Novel septin 9 repeat motifs altered in neuralgic amyotrophy bind and bundle microtubules. J Cell Biol 203:895–905
Bertin A, McMurray MA, Grob P, Park SS, Garcia G 3rd, Patanwala I, Ng HL, Alber T, Thorner J, Nogales E (2008) Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc Natl Acad Sci USA 105:8274–8279. https://doi.org/10.1073/pnas.0803330105
Bourne HR (1997) The arginine finger strikes again. Nature 389:673–674
Brausemann A, Gerhardt S, Schott AK, Einsle O, Große-Berkenbusch A, Johnsson N, Gronemeyer T (2016) Crystal structure of Cdc11, a septin subunit from Saccharomyces cerevisiae. J Struct Biol 193:157–161. https://doi.org/10.1016/j.jsb.2016.01.004
Bridges AA, Zhang H, Mehta SB, Occhipinti P, Tani T, Gladfelter AS (2014) Septin assemblies form by diffusion-driven annealing on membranes. Proc Natl Acad Sci USA 111(6):2146–2151. https://doi.org/10.1073/pnas.1314138111
Bridges AA, Jentzsch MS, Oakes PW, Occhipinti P, Gladfelter AS (2016) Micron-scale plasma membrane curvature is recognized by the septin cytoskeleton. J Cell Biol 213(1):23–32. https://doi.org/10.1083/jcb.201512029
Byers B, Goetsch L (1976) A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol 69(3):717–721
Damalio JCP, Garcia W, Alves Macêdo JN, de Almeida MI, Andreu JM, Giraldo R, Garratt RC, Ulian Araújo AP (2012) Self assembly of human septin 2 into amyloid filaments. Biochimie 94:628–636
Fung KYY, Dai L, Trimble WS (2014) Cell and molecular biology of septins. Inl Rev Cell Mol Biol 310:289–339
Garcia W, de Araújo APU, Oliveira Neto M, Ballestero MRM, Polikarpov I, Tanaka M, Tanaka T, Garratt RC (2006) Dissection of a human septin: definition and characterization of distinct domains within human SEPT4. Biochemistry 45(46):13918–13931
Garcia W, Ulian de Araújo AP, Lara F, Foguel D, Tanaka M, Tanaka T, Garratt RC (2007) An intermediate in the thermal unforlding of the GTPase domain of human septin 4 (SEPT4/Bradeion-β) forms amyloid filaments in vitro. Biochemistry 46:11101–11109
Garcia G 3rd, Finnigan GC, Heasley LR, Sterling SM, Aggarwal A, Pearson CG, Nogales E, McMurray MA, Thorner J (2016) Assembly, molecular organization, and membrane-binding properties of development-specific septins. J Cell Biol 212:515–529. https://doi.org/10.1083/jcb.201511029
Hartwell LH (1971) Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res 69:265–276
Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ (2010) A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329:436–439. https://doi.org/10.1126/science.1191054
Huang YW, Surka MC, Reynaud D, Pace-Asciak C, Trimble WS (2006) GTP binding and hydrolysis kinetics of human septin 2. FEBS J 273:3248–3260
Kim MS, Froese CD, Estey MP, Trimble WS (2011) SEPT9 occupies the terminal positions in septin octamers and mediates polymerization-dependent functions in abscission. J Cell Biol 195:815–826. https://doi.org/10.1083/jcb.201106131
Kinoshita M (2003) Assembly of mammalian septins. J Biochem 134:491–496
Kinoshita N, Kimura K, Matsumoto N, Watanabe M, Fukaya M, Ide C (2004) Mammalian septin Sept2 modulates the activity of GLAST, a glutamate transporter in astrocytes. Genes Cells 9:1–14
Lee KI, Im W, Pastor RW (2014) Langevin dynamics simulations of charged model phosphatidylinositol lipids in the presence of diffusion barriers: toward an atomic level understanding of corralling of PIP2 by protein fences in biological membranes. BMC Biophys 7:13. https://doi.org/10.1186/s13628-014-0013-3
Macara IG, Baldarelli R, Field CM, Glotzer M, Hayashi Y, Hsu SC, Kennedy MB, Kinoshita M, Longtine M, Low C, Maltais LJ, McKenzie L, Mitchison TJ, Nishikawa T, Noda M, Petty EM, Peifer M, Pringle JR, Robinson PJ, Roth D, Russell SE, Stuhlmann H, Tanaka M, Tanaka T, Trimble WS, Ware J, Zeleznik-Le NJ, Zieger B (2002) Mammalian septins nomenclature. Mol Biol Cell 13(12):4111–4113
Macedo JN, Valadares NF, Marques IA, Ferreira FM, Damalio JC, Pereira HM, Garratt RC, Araujo AP (2013) The structure and properties of septin 3: a possible missing link in septin filament formation. Biochem J 450:95–105. https://doi.org/10.1042/BJ20120851
Marques IA, Valadares NF, Garcia W, Damalio JC, Macedo JN, de Araújo AP, Botello CA, Andreu JM, Garratt RC (2012) Septin C-terminal domain interactions: implications for filament stability and assembly. Cell Biochem Biophys 62:317–328. https://doi.org/10.1007/s12013-011-9307-0
McMurray M (2014) Lean forward: Genetic analysis of temperature-sensitive mutants unfolds the secrets of oligomeric protein complex assembly. Bioessays 36:836–846
McMurray MA (2016) Assays for genetic dissection of septin filament assembly in yeast, from de novo folding through polymerization. Methods Cell Biol 136:99–116. https://doi.org/10.1016/bs.mcb.2016.03.012
Merk A, Bartesaghi A, Banerjee S, Falconieri V, Rao P, Davis MI, Pragani R, Boxer MB, Earl LA, Milne JL, Subramaniam S (2016) Breaking cryo-EM resolution barriers to facilitate drug discovery. Cell 165(7):1698–1707. https://doi.org/10.1016/j.cell.2016.05.040
Mostowy S, Cossart P (2012) Septins: the fourth component of the cytoskeleton. Nature Rev 13:183–194
Mostowy S, Bonazzi M, Hamon MA, Tham TN, Mallet A, Lelek M, Gouin E, Demangel C, Brosch R, Zimmer C, Sartori A, Konoshita M, Lecuit M, Cossart P (2010) Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe 8:433–444
Nagata K, Asano T, Nozawa Y, Inagaki M (2004) Biochemical and c cell biological analyses of a mammalian septin complex, Sept7/9b/11. J Biol Chem 279:55895–55904
Nakahira M, Macedo JN, Seraphim TV, Cavalcante N, Souza TA, Damalio JC, Reyes LF, Assmann EM, Alboghetti MR, Garratt RC et al (2010) A draft of the human septin interactome. PLoS One 5:e13799
Neubauer K, Zeiger B (2017a) The mammalian septin interactome. Front Cell Dev Biol 5:3. https://doi.org/10.3389/fcell.2017.00003
Neubauer K, Zeiger B (2017b) The mammalian septin interactome. Front Cell Dev Biol 5:1–9
Pan F, Malmberg RL, Momany M (2007) Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol Biol 7:103
Pinto APA, Pereira HM, Zeraik AE, Ciol H, Ferreira FM, Brandão-Neto J, DeMarco R, Navarro MVAS, Risi C, Galkin VE, Garratt RC, Araujo APU (2017) Filaments and fingers: Novel structural aspects of the single septin from Chlamydomonas reinhardtii. J Biol Chem 292(26):10899–10911. https://doi.org/10.1074/jbc.M116.762229
Sadian Y, Gastogiannis C, Patasi C, Hofnagel O, Goody RS, Farkasovsky M, Rausner S (2013) The role of Cdc42 and Gic1 in the regulation of septin filament formation and dissociation. eLife 2:e01085
Sala FA, Valadares NF, Macedo JN, Borges JC, Garratt RC (2016) Heterotypic coiled-coil formation is essential for the correct assembly of the septin heterofilament. Biophys J 111(12):2608–2619. https://doi.org/10.1016/j.bpj.2016.10.032
Sandrock K, Bartsch I, Blaser S, Busse A, Busse E, Zieger B (2011) Characterization of human septin interactions. Biol Chem 392:751–761
Sellin ME, Sandblad L, Stenmark S, Gullberg M (2011) Deciphering the rules governing assembly order of mammalian septin complexes. Mol Biol Cell. 22:3152–3164. https://doi.org/10.1091/mbc.E11-03-0253
Serrão VH, Alessandro F, Caldas VE, Marçal RL, Pereira HD, Thiemann OH, Garratt RC (2011) Promiscuous interactions of human septins: the GTP binding domain of SEPT7 forms filaments within the crystal. FEBS Lett 585:3868–3873. https://doi.org/10.1016/j.febslet.2011.10.043
Sirajuddin M, Farkasovsky M, Hauer F, Kühlmann D, Macara IG, Weyand M, Stark H, Wittinghofer A (2007) Structural insight into filament formation by mammalian septins. Nature 449:311–315
Sirajuddin M, Farkasovsky M, Zent E, Wittinghofer A (2009) GTP-induced conformational changes in septins and implications for function. Proc Natl Acad Sci USA. 106:16592–16597. https://doi.org/10.1073/pnas.0902858106
Smith C, Dolat L, Angelis D, Forgacs E, Spiliotis ET, Galkin VE (2015) Septin 9 exhibits polymorphic binding to F-actin and inhibits myosin and cofilin activity. J Mol Biol 427:3273–3284
Souza TA, Barbosa JA (2010) Cloning, overexpression, purification and preliminary characterization of human septin 8. Protein J 29:328–335
Surka MC, Tsang CW, Trimble WS (2002) The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell 13:3532–3545
Valadares NF, Garratt RC (2016) Septin crystallization for structural analysis. Methods Cell Biol 136:321–338. https://doi.org/10.1016/bs.mcb.2016.03.017
Weems A, McMurray M (2017) The step-wise pathway of septin hetero-octamer assembly in budding yeast. Elife 25:6. https://doi.org/10.7554/eLife.23689
Weirich CS, Erzberger JP, Barral Y (2008) The septin family of GTPases: achitecture and dynamics. Nat Rev 9:478–489
Wittinghofer A, Pai EF (1991) The structure of Ras protein: a model for a universal molecular switch. Trends Biochem Sci 16:382–387
Zent E, Wittinghofer A (2014) Human septin isoforms and the GDP-GTP cycle. Biol Chem 395(2):169–180. https://doi.org/10.1515/hsz-2013-0268
Zent E, Vetter I, Wittinghofer A (2011) Structural and biochemical properties of Sept7, a unique septin required for filament formation. Biol Chem 392:791–797. https://doi.org/10.1515/BC.2011.082
Zeraik AE, Pereira HM, Santos YV, Brandão-Neto J, Spoerner M, Santos MS, Colnago LA, Garratt RC, Araújo AP, DeMarco R (2014) Crystal structure of a Schistosoma mansoni septin reveals the phenomenon of strand slippage in septins dependent on the nature of the bound nucleotide. J Biol Chem 289:7799–7811. https://doi.org/10.1074/jbc.M113.525352
Zeraik AE, Staykova M, Fontes MG, Nemuraitė I, Quinlan R, Araújo AP, DeMarco R (2016) Biophysical dissection of schistosome septins: Insights into oligomerization and membrane binding. Biochimie 131:96–105. https://doi.org/10.1016/j.biochi.2016.09.014
Zhang J, Kong C, Xie H, McPherson PS, Grinstein S, Trimble WS (1999a) Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr Biol 9:1458–1467
Zhang B, Zhang Y, Collins CC, Johnson DI, Zheng Y (1999b) A built-in arginine finger triggers the self-stimulatory GTPase-activating activity of rho family GTPases. J. Biol Chem 274:2609–2612
Acknowledgements
We would like to acknowledge the financial support of the Brazilian funding agencies CNPq, FAPESP and FAPDF. We would also like to specifically mention the dedication of students and post-docs over the last 10 years (too many to mention by name, but they know who they are) who have contributed significantly to the structural biology of septins.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Napoleão Fonseca Valadares declares that he has no conflicts of interest. Humberto d´Muniz Pereira declares that he has no conflicts of interest. Ana Paula Ulian de Araujo declares that she has no conflicts of interest. Richard Charles Garratt declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Additional information
This article is part of a Special Issue on ‘Latin America’ edited by Pietro Ciancaglini and Rosangela Itri.
Rights and permissions
About this article
Cite this article
Valadares, N.F., d’ Muniz Pereira, H., Ulian Araujo, A.P. et al. Septin structure and filament assembly. Biophys Rev 9, 481–500 (2017). https://doi.org/10.1007/s12551-017-0320-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-017-0320-4