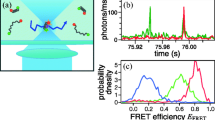
Abstract
Single-molecule detection (SMD) with fluorescence is a widely used microscopic technique for biomolecule structure and function characterization. The modern light microscope with high numerical aperture objective and sensitive CCD camera can image the brightly emitting organic and fluorescent protein tags with reasonable time resolution. Single-molecule imaging gives an unambiguous bottom-up biomolecule characterization that avoids the “missing information” problem characteristic of ensemble measurements. It has circumvented the diffraction limit by facilitating single-particle localization to ∼1 nm. Probes developed specifically for SMD applications extend the advantages of single-molecule imaging to high probe density regions of cells and tissues. These applications perform under conditions resembling the native biomolecule environment and have been used to detect both probe position and orientation. Native, high density SMD may have added significance if molecular crowding impacts native biomolecule behavior as expected inside the cell.






Similar content being viewed by others
Abbreviations
- AOM:
-
Acousto-optic modulator
- AS:
-
Activatable shutter
- BFP:
-
Back focal plane
- BS:
-
Beam stop
- CCD:
-
Charge-coupled device
- Cys707:
-
Myosin head domain highly reactive thiol
- DM:
-
Dichroic mirror
- ELC:
-
Myosin essential light chain
- GFP:
-
Green fluorescent protein
- HCRLC:
-
Human cardiac myosin regulatory light chain
- NA:
-
Numerical aperture
- NSOM:
-
Near-field scanning optical microscope
- PA:
-
Photoactivatable
- PA-FP:
-
Photoactivatable fluorescent protein
- PA-GFP:
-
Photoactivatable green fluorescent protein
- PALM:
-
Photoactivated localization microscopy
- PC:
-
Pockels cell
- PEG:
-
Polyethylene glycol
- PMMA:
-
Polymethylmethacrylate
- PSF:
-
Point spread function
- RLC:
-
Myosin regulatory light chain
- S1:
-
Myosin subfragment 1
- SAF:
-
Supercritical angle fluorescence
- SI:
-
Structured illumination
- SMD:
-
Single-molecule detection
- STED:
-
Stimulated emission depletion
- STORM:
-
Stochastic optical reconstruction microscopy
- TIRF:
-
Total internal reflection microscopy
References
Arata T (1990) Orientation of spin-labeled light chain 2 of myosin heads in muscle fibers. J Mol Biol 214:471–478
Ashkin A, Dziedzic JM, Yamane T (1987) Optical trapping and manipulation of single cells using infrared laser beams. Nature 330:769–771
Axelrod D (1981) Cell-substrate contacts illuminated by total internal reflection fluorescence. J Cell Biol 89:141–145
Axelrod D (2001) Total internal reflection fluorescence microscopy in cell biology. Traffic 2:764–774
Axelrod D, Thompson NL, Burghardt TP (1983) Total internal reflection flourescence microscopy. J Microsc 129:19–28
Bates M, Blosser TR, Zhuang X (2005) Short-range spectroscopic ruler based on a single-molecule optical switch. Phys Rev Lett 94:108101
Betzig E, Chichester RJ (1993) Single molecules observed by near-field scanning optical microscopy. Science 262:1422–1425
Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF (2006) Imaging intracellular fluorescent proteins at nanometer resolution. Science 313:1642–1645
Bobroff N (1986) Position measurement with a resolution and noise-limited instrument. Rev Sci Instrum 57:1152–1157
Borejdo J, Ushakov DS, Akopova I (2002) Regulatory and essential light chains of myosin rotate equally during contraction of skeletal muscle. Biophys J 82:3150–3159
Borejdo J, Talent J, Akopova I, Burghardt TP (2006) Rotations of a few cross-bridges in muscle by confocal total internal reflection microscopy. Biochim Biophys Acta 1763:137–140
Brejc K, Sixma TK, Kitts PA, Kain SR, Tsien RY, Ormo M, Remington SJ (1997) Structural basis for dual excitation and photoisomerization of the Aequorea victoria green fluorescent protein. Proc Natl Acad Sci USA 94:2306–2311
Burghardt TP, Axelrod D (1981) Total internal reflection/fluorescence photobleaching recovery study of serum albumin adsorption dynamics. Biophys J 33:455–467
Burghardt TP, Ajtai K, Borejdo J (2006a) In situ single molecule imaging with attoliter detection using objective total internal reflection confocal microscopy. Biochemistry 45:4058–4068
Burghardt TP, Charlesworth JE, Halstead MF, Tarara JE, Ajtai K (2006b) In situ fluorescent protein imaging with metal film-enhanced total internal reflection microscopy. Biophys J 90:4662–4671
Burghardt TP, Hipp AP, Ajtai K (2009a) Around-the-objective total internal reflection fluorescence microscopy. Appl Opt 48:6120–6131
Burghardt TP, Li J, Ajtai K (2009b) Single myosin lever-arm orientation in a muscle fiber detected with photoactivatable GFP. Biochemistry 48:754–765
Corrie JET, Brandmeier BD, Ferguson RE, Trentham DR, Kendrick-Jones J, Hopkins SC, van der Heide UA, Goldman YE, Sabido-David C, Dale RE, Criddle S, Irving M (1999) Dynamic measurement of myosin light-chain-domain tilt and twist in muscle contraction. Nature 400:425–430
Donnert G, Keller J, Medda R, Andrei MA, Rizzoli SO, Luhrmann R, Jan R, Eggeling C, Hell SW (2006) Macromolecular-scale resolution in biological fluorescence microscopy. Proc Natl Acad Sci USA 103:11440–11445
Eisenberg E, Hill TL (1985) Muscle contraction and free energy transduction in biological systems. Science 227:999–1006
Finer JT, Simmons RM, Spudich JA (1994) Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368:113–119
Fulbright RM, Axelrod D (1993) Dynamics of nonspecific adsorption of insulin of erythrocyte membranes. J Fluoresc 3:1–16
Geeves MA, Holmes KC (1999) Structural mechanism of muscle contraction. Annu Rev Biochem 68:687–728
Gugel H, Bewersdorf J, Jakobs S, Engelhardt J, Storz R, Hell SW (2004) Cooperative 4Pi excitation and detection yields sevenfold sharper optical sections in live-cell microscopy. Biophys J 87:4146–4152
Gustafsson MGL (2005) Nonlinear structured-illumination microscopy: wide field fluorescence imaging with theoretically unlimited resolution. Proc Natl Acad Sci USA 102:13081–13086
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Harrick NJ (1979) Internal reflection spectroscopy. Wiley Interscience, New York
Hellen EH, Axelrod D (1987) Fluorescence emission at dielectric and metal-film interfaces. J Opt Soc Am B 4:337–350
Hirschfeld T (1976) Optical microscopic observation of single small molecules. Appl Opt 15:2965–2966
Holtzer L, Meckel T, Schmidt T (2007) Nanometric three-dimensional tracking of individual quantum dots in cells. Appl Phys Lett 90:053902-1–053902-3
Huxley HE (1969) The mechanism of muscular contraction. Science 164:1356–1366
Inoué S, Shimomura O, Goda M, Shribak M, Tran PT (2002) Fluorescence polarization of green fluorescence protein. Proc Natl Acad Sci USA 99:4272–4277
Irving M, Allen TSC, Sabido-David C, Cralk JS, Brandmeler B, Kendrick-Jones J, Corrie JET, Trentham DR, Goldman YE (1995) Tilting of the light-chain region of myosin during step length changes and active force generation in skeletal muscle. Nature 375:688–690
Ishijima A, Doi T, Sakurada K, Yanagida T (1991) Sub-piconewton force fluctuations of actomyosin in vitro. Nature 352:301–306
Ishijima A, Kojima H, Funatsu T, Tokunaga M, Higuchi H, Tanaka H, Yanagida T (1998) Simultaneous observation of individual ATPase and mechanical events by a single myosin molecule during interaction with actin. Cell 92:161–171
Katz A (1967) Principles of statistical mechanics: the information theory approach. Freeman, San Francisco, pp 14–22
Kishino A, Yanagida T (1988) Force measurements by micromanipulation of a single actin filament by glass needles. Nature 334:74–76
Levene MJ, Korlach J, Turner SW, Foquet M, Craighead HG, Webb WW (2003) Zero-mode waveguides for single-molecule analysis at high concentrations. Science 299:682–686
Lewis A, Isaacson M, Harootunian A, Muray A (1984) Development of a 500 Å spatial resolution light microscope. I. Light is efficiently transmitted through λ/16 diameter apertures. Ultramicroscopy 13(3):227–231
Lippincott-Schwartz J, Patterson GH (2003) Development and use of fluorescent protein markers in living cells. Science 300:87–91
Lord SJ, Conley NR, Lee HL, Samuel R, Liu N, Twieg RJ, Moerner WE (2008) A photoactivatable push−pull fluorophore for single-molecule imaging in live cells. J Am Chem Soc 130(29):9204–9205
Minton AP (2001) The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J Biol Chem 276:10577–10580
Moerner WE, Kador L (1989) Optical detection and spectroscopy of single molecules in a solid. Phys Rev Lett 62:2535–2538
Mortensen KI, Churchman LS, Spudich JA, Flyvbjerg H (2010) Optimized localization analysis for single-molecule tracking and super-resolution microscopy. Nat Meth 7:377–381
Muthu P, Mettikolla P, Calander N, Luchowski R, Gryczynski I, Gryczynski Z, Szczesna-Cordary D, Borejdo J (2010) Single molecule kinetics in the familial hypertrophic cardiomyopathy D166V mutant mouse heart. J Mol Cell Cardiol 48(5):989–998
Neher E, Sakmann B (1976) Single channel currents recorded from membrane of denervated frog muscle fibres. Nature 260:799–802
Neuweiler H, Sauer M (2005) Exploring life by single-molecule fluorescence spectroscopy. Anal Chem 77:179A–185A
Nguyen DC, Keller RA, Jett JH, Martin JC (1987) Detection of single molecules of phycoerythrin in hydrodynamically focused flows by laser-induced fluorescence. Anal Chem 59:2158–2161
Ober RJ, Ram S, Ward ES (2004) Localization accuracy in single-molecule microscopy. Biophys J 86:1185–1200
Orrit M, Bernard J (1990) Single pentacene molecules detected by fluorescence excitation in a p-terphenyl crystal. Phys Rev Lett 65:2716–2719
Patterson GH, Lippincott-Schwartz J (2002) A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297:1873–1877
Pavani SRP, Thompson MA, Biteen JS, Lord SJ, Liu N, Twieg RJ, Piestun R, Moerner WE (2009) Three-dimensional, single-molecule fluorescence imaging beyond the diffraction limit by using a double-helix point spread function. Proc Natl Acad Sci USA 106(9):2995–2999
Peck K, Stryer L, Glazer AN, Mathies RA (1989) Single-molecule fluorescence detection: autocorrelation criterion and experimental realization with phycoerythrin. Proc Natl Acad Sci USA 86:4087–4091
Peyser YM, Shaya S, Ajtai K, Burghardt TP, Muhlrad A (2003) Cosolvent induced aggregation inhibits myosin ATPase activity by stabilizing the predominant transition intermediate. Biochemistry 42:12669–12675
Pierobon P, Achouri S, Courty S, Dunn AR, Spudich JA, Dahan M, Cappello G (2010) Velocity, processivity, and individual steps of single myosin V molecules in live cells. Biophys J 96(10):4268–4275
Pohl DW, Denk W, Lanz M (1984) Optical stethoscopy: image recording with resolution λ/20. Appl Phys Lett 44:651–653
Ram S, Prabhat P, Chao J, Ward ES, Ober RJ (2008) High accuracy 3D quantum dot tracking with multifocal plane microscopy for the study of fast intracellular dynamics in live cells. Biophys J 95:6025–6043
Rocheleau JV, Edidin M, Piston DW (2003) Intrasequence GFP in class I MHC molecules, a rigid probe for fluorescence anisotropy measurements of the membrane environment. Biophys J 84:4078–4086
Rosell FI, Boxer SG (2003) Polarized absorption spectra of green fluorescent protein single crystals: transition dipoles moment directions. Biochemistry 42:177–183
Ruckstuhl T, Seeger S (2003) Confocal total-internal-reflection fluorescence microscopy with a high-aperture parabolic mirror lens. Appl Opt 42:3277–3283
Ruckstuhl T, Seeger S (2004) Attoliter detection volumes by confocal total-internal-reflection fluorescence microscopy. Opt Lett 29:569–571
Ruckstuhl T, Verdes D (2004) Supercritical angle fluorescence (SAF) microscopy. Opt Express 12:4246–4254
Rust MJ, Bates M, Zhuang X (2006) Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat Meth 3:793–795
Simmons RM, Finer JT, Chu S, Spudich JA (1996) Quantitative measurements of force and displacement using an optical trap. Biophys J 70:1813–1822
Stoneking MR, Den Hartog DJ (1997) Maximum-likelihood fitting of data dominated by Poisson statistical uncertainties. Rev Sci Instrum 68:914–917
Thompson NL, Burghardt TP, Axelrod D (1981) Measuring surface dynamics of biomolecules by total internal reflection fluorescence with photobleaching recovery and correlation spectroscopy. Biophys J 33:435–454
Thompson RE, Larson DR, Webb WW (2002) Precise nanometer localization analysis for individual fluorescent probes. Biophys J 82:2775–2783
Tikunov BA, Sweeney HL, Rome LC (2001) Quantitative electrophoretic analysis of myosin heavy chains in single muscle fibers. J Appl Physiol 90:1927–1935
Timasheff SN (2002) Protein-solvent preferential interactions, protein hydration, and the modulation of biochemical reactions by solvent components. Proc Natl Acad Sci USA 99:9721–9726
Wagner PD, Weeds AG (1977) Studies on the role of myosin alkali light chains. Recombination and hybridization of light chains and heavy chains in subfragment one preparations. J Mol Biol 109:455–470
Whitten WB, Ramsey JM, Arnold S, Bronk BV (1991) Single-molecule detection limits in levitated microdroplets. Anal Chem 63:1027–1031
Yanagida T, Nakase M, Nishiyama K, Oosawa F (1984) Direct observation of motion of single F-actin filaments in the presence of myosin. Nature 307:58–60
Yanagida T, Harada Y, Ishijima A (1993) Nano-manipulation of actomyosin molecular motors in vitro: a new working principle. Trends Biochem Sci 18:319–324
Yildiz A, Forkey JN, McKinney SA, Ha T, Goldman YE, Selvin PR (2003) Myosin V walks hand-over-hand: single fluorophore imaging with 1.5-nm localization. Science 300(5628):2061–2065
Acknowledgments
The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant R01AR049277; the National Heart, Lung, Blood Institute (NHLBI) grant R01HL095572; and the Mayo Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burghardt, T.P., Ajtai, K. Single-molecule fluorescence characterization in native environment. Biophys Rev 2, 159–167 (2010). https://doi.org/10.1007/s12551-010-0038-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-010-0038-z