Abstract
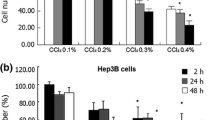
Leucocytes are susceptible to the toxic effects of deoxynivalenol (DON), which is a trichothecene mycotoxin produced by a number of fungi including Fusarium species. One mechanism of action is mediated by reactive oxygen species (ROS). The liver is an important target for toxicity caused by foreign compounds including mycotoxins. On the other hand, little is known about the influence of the redox state on hepatocytes treated with DON. The present study investigated the effect of DON on the cytosolic redox state and antioxidative system in the human hepatoma cell line HepG2. The cell viability of human monocyte cell line THP-1 or leukemia cell line KU812 treated with 2.5 and 5 μmol/l DON were significantly reduced. However, HepG2 cells showed no toxic effects under the same conditions and did not exhibit an increased oxidative state. Further experiments showed that thioredoxin-1 (Trx-1) protein levels but not glutathione increased in the cells treated with 10 μmol/l DON. In addition, the enhancement of Trx-1 content was repressed by antioxidants. These results suggest that DON-induced accumulation of Trx-1 in HepG2 cells plays one of the key roles in protection against cytotoxicity caused by DON and that the mechanism may be mediated by the antioxidant properties of Trx-1.




Similar content being viewed by others

References
Abbas HK, Mirocha CJ, Rosiles R, Carvajal M (1998) Decomposition of zearalenone and deoxynivalenol in the process of making tortillas from corn. Cereal Chem 65:15–19
Ago T, Sadoshima J (2006) Thioredoxin and ventricular remodeling. J Mol Cell Cardiol 41:762–773
Bennett JW, Klich M (2003) Mycotoxins. Clin Microbiol Rev 16:497–516
Bouaziz C, Abid-Essefi S, Bouslimi A, El Golli E, Bacha H (2006) Cytotoxicity and related effects of T-2 toxin on cultured Vero cells. Toxicon 48:343–352
Bouaziz C, Sharaf El Dein O, El Golli E, Abid-Essefi S, Brenner C, Lemaire C, Bacha H (2008) Different apoptotic pathways induced by zearalenone, T-2 toxin and ochratoxin A in human hepatoma cells. Toxicology 254:19–28
Braicu C, Berindan-Neagoe I, Tudoran O, Balacescu O, Rugina D, Gherman C, Socaciu C, Irimie A (2009) In vitro evaluation of the chemoprotective action of flavan-3-ols against deoxynivalenol related toxicity. Arch Zootech 12:45–55
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Fath MA, Ahmad IM, Smith CJ, Spence J, Spitz DR (2011) Enhancement of carboplatin-mediated lung cancer cell killing by simultaneous disruption of glutathione and thioredoxin metabolism. Clin Cancer Res 17:6206–6217
Gaubin Y, Vaissade F, Croute F, Beau B, Soleilhavoup JP, Murat JC (2000) Implication of free radicals and glutathione in the mechanism of cadmium-induced expression of stress proteins in the A549 human lung cell-line. Biochim Biophys Acta 1495:4–13
Go YM, Kang SM, Roede JR, Orr M, Jones DP (2011) Increased inflammatory signaling and lethality of influenza H1N1 by nuclear thioredoxin-1. PLoS One 6:e18918
Gouze ME, Laffitte J, Rouimi P, Loiseau N, Oswald IP, Galtier P (2006) Effect of various doses of deoxynivalenol on liver xenobiotic metabolizing enzymes in mice. Food Chem Toxicol 44:476–483
Grant CM, MacIver FH, Dawes IW (1996) Glutathione is an essential metabolite required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. Curr Genet 29:511–515
Kagaya N, Tagawa Y, Nagashima H, Saijo R, Kawase M, Yagi K (2002) Suppression of cytotoxin-induced cell death in isolated hepatocytes by tea catechins. Eur J Pharmacol 450:231–236
Krishnaswamy R, Devaraj SN, Padma VV (2010) Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: Prevention of NF-[kappa] B nuclear localization and down regulation of NF-[kappa] B and Cyclo-Oxygenase-2 expression. Free Radic Biol Med 49:50–60
Kushiro M (2008) Effects of milling and cooking processes on the deoxynivalenol content in wheat. Int J Mol Sci 9:2127–2145
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Marzocco S, Russo R, Bianco G, Autore G, Severino L (2009) Pro-apoptotic effects of nivalenol and deoxynivalenol trichothecenes in J774A.1 murine macrophages. Toxicol Lett 189:21–26
Nielsen C, Lippke H, Didier A, Dietrich R, Martlbauer E (2009a) Potential of deoxynivalenol to induce transcription factors in human hepatoma cells. Mol Nutr Food Res 53:479–491
Nielsen C, Casteel M, Didier A, Dietrich R, Märtlbauer E (2009b) Trichothecene-induced cytotoxicity on human cell lines. Mycotoxin Res 25:77–84
Nowicki TW, Gaba WD, Dexter JE, Matsuo RR, Clear RM (1988) Retention of the Fusarium mycotoxin deoxynivalenol in wheat during processing and cooking of spaghetti and noodles. J Cereal Sci 8:189–202
Ohashi S, Nishio A, Nakamura H, Kido M, Ueno S, Uza N, Inoue S, Kitamura H, Kiriya K, Asada M, Tamaki H, Matsuura M, Kawasaki K, Fukui T, Watanabe N, Nakase H, Yodoi J, Okazaki K, Chiba T (2006) Protective roles of redox-active protein thioredoxin-1 for severe acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 290:G772–G781
Pestka JJ (2008) Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25:1128–1140
Pestka JJ, Uzarski RL, Islam Z (2005) Induction of apoptosis and cytokine production in the Jurkat human T cells by deoxynivalenol: role of mitogen-activated protein kinases and comparison to other 8-ketotrichothecenes. Toxicology 206:207–219
Rahman I, MacNee W (1999) Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am J Physiol 277:L1067–L1088
Rocha O, Ansari K, Doohan FM (2005) Effects of trichothecene mycotoxins on eukaryotic cells: a review. Food Addit Contam 22:369–378
Schoettler S, Bascope M, Sterner O, Anke T (2006) Isolation and characterization of two verrucarins from Myrothecium roridum. Z Naturforsch C 61:309–314
Sugita-Konishi Y, Pestka JJ (2001) Differential upregulation of TNF-alpha, IL-6, and IL-8 production by deoxynivalenol (vomitoxin) and other 8-ketotrichothecenes in a human macrophage model. J Toxicol Environ Health A 64:619–636
Sugiyama K, Izawa S, Inoue Y (2000) The Yap1p-dependent induction of glutathione synthesis in heat shock response of Saccharomyces cerevisiae. J Biol Chem 275:15535
Sugiyama K, Muroi M, Tanamoto K, Nishijima M, Sugita-Konishi Y (2010) Deoxynivalenol and nivalenol inhibit lipopolysaccharide-induced nitric oxide production by mouse macrophage cells. Toxicol Lett 192:150–154
Sugiyama K, Kinoshita M, Kamata Y, Minai Y, Sugita-Konishi Y (2011) (-)-Epigallocatechin gallate suppresses the cytotoxicity induced by trichothecene mycotoxins in mouse cultural macrophages. Mycotoxin Res 1-5
Tian C, Gao P, Zheng Y, Yue W, Wang X, Jin H, Chen Q (2008) Redox status of thioredoxin-1 (TRX1) determines the sensitivity of human liver carcinoma cells (HepG2) to arsenic trioxide-induced cell death. Cell Res 18:458–471
Vincenzini MT, Marraccini P, Iantomasi T, Favilli F, Pacini S, Ruggiero M (1993) Altered metabolism of glutathione in cells transformed by oncogenes which cause resistance to ionizing radiations. FEBS Lett 320:219–223
Watson WH, Yang X, Choi YE, Jones DP, Kehrer JP (2004) Thioredoxin and its role in toxicology. Toxicol Sci 78:3–14
Zhang X, Jiang L, Geng C, Cao J, Zhong L (2009) The role of oxidative stress in deoxynivalenol-induced DNA damage in HepG2 cells. Toxicon 54:513–518
Acknowledgements
This work was supported by a Health and Labor Sciences Research Grant from the Ministry of Health, Labor and Welfare of Japan. The authors are also grateful to Rino Yamazaki for her excellent technical assistance.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugiyama, Ki., Kinoshita, M., Kamata, Y. et al. Thioredoxin-1 contributes to protection against DON-induced oxidative damage in HepG2 cells. Mycotoxin Res 28, 163–168 (2012). https://doi.org/10.1007/s12550-012-0128-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-012-0128-9