Abstract
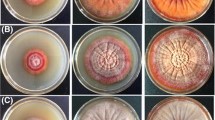
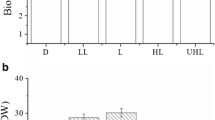
Light has a profound influence on ochratoxin biosynthesis by Penicillia. When incubated under constant daylight of a certain intensity, ochratoxin A biosynthesis is decreased by about 20–30% compared to incubation under constant darkness. Under day/night oscillation, the ochratoxin A polyketide synthase gene, a key gene of the ochratoxin A biosynthesis pathway, is rhythmically expressed, and moreover, the amount of ochratoxin also oscillates between the amounts produced either during constant darkness or during constant light. This indicates a partial degradation of ochratoxin A (20–30%) under light conditions until a certain lower limit is reached. This behavior is dependent on the light intensity. At 1,600 Lux, only weak effects could be observed; however, at 2,800 Lux, the effects became significant. After growth under constant light conditions, Penicillium produced ochratoxin B at amounts which are 5 times higher than after growth in constant dark or in alternating light/dark conditions. Growth experiments in the dark on medium with increasing amounts of ochratoxin A revealed that externally applied ochratoxin is moderately toxic. However, if the same growth experiments are carried out under light conditions, the growth inhibiting activity of ochratoxin A is greatly increased, indicating that light amplifies the toxic activity of ochratoxin. Because of the oscillation of the concentration of ochratoxin A during night and day incubation, Penicillium seems to have developed an adaptive mechanism to reduce the amount of ochratoxin A during daylight below a toxic level.





Similar content being viewed by others
References
Adams TH, Wieser JK, Yu JH (1998) Asexual sporulation in Aspergillus nidulans. Microbiol Mol Biol Rev 35–54
Bayram Ö, Krappmann S, Ni M, Bok JW, Helmstaedt K, Valerius O, Braus-Stromeyer S, Kwon NJ, Keller NP, Yu JH, Braus GH (2008) VeIB/VeA/LaeA Complex coordinates light signal with fungal development and secondary metabolism. Science 320:1504–1506
Belli N, Ramos AJ, Sanchis V, Marin S (2006) Effect of photoperiod and day-night temperatures simulating field conditions on growth and ochratoxin A production of Aspergillus carbonarius strains isolated from grapes. Food Microbiol 23:622–627
Blumenstein A, Vienken K, Tasler R, Purschwitz J, Veith D, Frankenberg-Dinkel N, Fischer R (2005) The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr Biol 15:1833–1838
Briggs WR, Huala E (1999) Blue-light photoreceptors in higher plants. Annu Rev Cell Dev Biol 15:33–62
Cerdá-Olmedo E (2001) Phycomyces and the biology of light and colour. FEMS Microbiol Rev 25:503–512
Corrochano LM (2002) Photomorphogenesis in Phycomyces: differential display of gene expression by PCR with arbitrary primers. Mol Gen Genom 267:424–428
Dunlap JC (1993) Genetic analysis of circadian clocks. An Rev Physiol 55:683–728
Dunlap JC, Loros JJ (2006) How fungi keep time: circadian system in Neurospora and other fungi. Curr Opin Microbiol 9:579–587
Fankhauser C (2001) The phytochromes, a family of red/far-red absorbing photoreceptors. J Biol Chem 276:11453–11456
Froehlich AC, Liu Y, Loros JJ, Dunlap JC (2002) White collar-1, a circadian blue light photoreceptor, binding to the frequency promotor. Science 297:815–819
Gillman IG, Yezek JM, Manderville RA (1998) Ochratoxin A acts as a photoactivatable DNA cleaving agent. Chem Commun 647–648
Häggblom P, Unestam T (1997) Blue light inhibits mycotoxin production and increases total lipids and pigmentation in Alternaria alternata. Appl Environ Microbiol 38:1074–1077
Höhler D (1998) Ochratoxin A in food and feed: occurrence, legislation and mode of action. Z Ernährungswiss 37:2–12
Idnurm A, Rodríguez-Romero J, Corrochano LM, Sanz C, Iturriaga EA, Eslava AP, Heitman J (2006) The Phycomyces madA gene encodes a blue-light photoreceptor for phototropism and other light responses. Proc Natl Acad Sci USA 103:4546–4551
Kato N, Brooks W, Calvo AM (2003) The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot Cell 2:1178–1186
Lee K, Singh P, Chung WC, Ash J, Kim TS, Hang L, Park S (2006) Light regulation of asexual development in the rice blast fungus, Magnaporthe oryzae. Fungal Gen Biol 43:694–706
Linden H, Ballario P, Macino G (1997) Blue light regulation in Neurospora crassa. Fungal Genet Biol 22:141–150
Lund F, Frisvad JC (2003) Penicillium verrucosum in wheat and barley indicates presence of ochratoxin A. J Appl Microbiol 95:1117–1123
Purschwitz J, Müller S, Kastner C, Fischer R (2006) Seeing the rainbow: light sensing in fungi. Curr Opin Microbiol 9:566–571
Purschwitz J, Müller S, Kastner C, Schöser M, Haas H, Espeso EA, Atoui A, Calvo AM, Fischer R (2008) Functional and physical interaction of blue- and red light sensors in Aspergillus nidulans. Curr Biol 18:1–5
Stander MA, Bornscheuer UT, Henke E, Steyn PS (2000a) Screening of commercial hydrolases for the degradation of ochratoxin A. J Agric Food Chem 48:5736–5739
Stander MA, Steyn PS, Lübben A, Miljkovic A, Mantle PG, Marais GJ (2000b) Influence of halogen salts on the production of the ochratoxins by Aspergillus ochraceus Wilh. J Agric Food Chem 48:1865–1871
Stinnett SM, Espeso EA, Cobeno L, Araújo-Bazán L, Calvo AM (2007) Aspergillus nidulans VeA subecllular localization is dependent on the importin α carrier and on light. Mol Microbiol 63:242–255
Yu JH, Keller N (2005) Regulation of secondary metabolism in filamentous fungi. Annu Rev Phytopathol 43:437–458
Acknowledgement
We would like Nicole Mischke and Michaela Ebli for excellent technical assistance. This work was financially supported by EC KBBE-2007-222690-2 MYCORED
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt-Heydt, M., Bode, H., Raupp, F. et al. Influence of light on ochratoxin biosynthesis by Penicillium . Mycotox Res 26, 1–8 (2010). https://doi.org/10.1007/s12550-009-0034-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-009-0034-y