Abstract
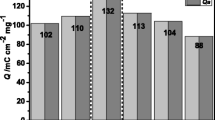
Vanadium pentoxide (V2O5) film was used to fabricate an electrochromic device counterbalanced with a dry-deposited ion storage layer (ISL). For the ISL, a mixture of antimony-doped tin oxide (ATO) and Prussian blue (PB) powders (PB-ATO; 1:4 vol%) was dry deposited to form PB-ATO ISL as the counter electrode of the device, thereby improving electrochromic device characteristics. Dry deposition provided a film with a rough surface, which resulted in improved electrochromic properties because of the large surface area of the ISL, which provided more paths for electron movement. The ISL was deposited using a dry deposition system (i.e., the nanoparticle deposition system [NPDS]) to maximize the surface area of the film. The polyol method was used to fabricate V2O5 film with high crystallinity for better electrochromic properties. The fabricated V2O5-based electrochromic device (ECD) was evaluated by electrochemical analysis. Compared with the ECD without PB-ATO ISL, the difference in reflectance exceeded 50% for the ECD with PB-ATO ISL. Moreover, the ECD without ISL had a diffusion coefficient of 1.88 × 10−10 S2/s, while the ECD with PB-ATO ISL had an order of magnitude higher diffusion coefficient of 2.47 × 10−9 cm2/s. Therefore, the performance of the ECD was improved using dry-deposited ISL composed of PB-ATO. The coloration of the device was dramatically improved using PB-ATO ISL, compared with the device with ATO alone or without any ISL.










Similar content being viewed by others
References
Habib, M. A. (1992). Electrochromism. In O. J. Murphy, S. Srinivasan, & B. E. Conway (Eds.), Electrochemistry in transition: From the 20th to 21st century (pp. 51–62). Springer.
Mjejri, I., Gaudon, M., Song, G., Labrugere, C., & Rougier, A. (2018). Crystallized V2O5 as oxidized phase for unexpected multicolor electrochromism in V2O3 thick film. ACS Applied Energy Materials, 1(6), 2721–2729. https://doi.org/10.1021/acsaem.8b00386
McColl, K., & Cora, F. (2019). Phase stability of intercalated V2O5 battery cathodes elucidated through the Goldschmidt tolerance factor. Physical Chemistry Chemical Physics, 21(15), 7732–7744. https://doi.org/10.1039/c9cp00170k
Bae, J.-W., Koo, B.-R., & Ahn, H.-J. (2019). Fe doping effect of vanadium oxide films for enhanced switching elect performances. Ceramics International, 45(6), 7137–7142. https://doi.org/10.1016/j.ceramint.2018.12.219
Lu, Y.-R., Wu, T.-Z., Chen, C.-L., Wei, D.-H., Chen, J.-L., Chou, W.-C., & Dong, C.-L. (2015). Mechanism of electrochemical deposition and coloration of electrochromic V2O5 nano thin films: An in situ X-ray spectroscopy study. Nanoscale Research Letters, 10(1), 387. https://doi.org/10.1186/s11671-015-1095-9
Ozer, N. (1997). Electrochemical properties of sol-gel deposited vanadium pentoxide films. Thin Solid Films, 305(1), 80–87. https://doi.org/10.1016/S0040-6090(97)00086-2
Lee, J., Badie, S., Srimuk, P., Ridder, A., Shim, H., Choudhury, S., Nah, Y.-C., & Presser, V. (2018). Electrodeposition of hydrated vanadium pentoxide on nanoporous carbon cloth for hybrid energy storage. Sustainable Energy & Fuels, 2(3), 577–588. https://doi.org/10.1039/c7se00559h
Choi, D., Lee, M., Kim, H., Chu, W.-S., Chun, D.-M., Ahn, S.-H., & Lee, C. S. (2018). Investigation of dry-deposited ion storage layers using various oxide particles to enhance electrochromic performance. Solar Energy Materials and Solar Cells, 174, 599–606. https://doi.org/10.1016/j.solmat.2017.10.001
Chen, K.-C., Hsu, C.-Y., Hu, C.-W., & Ho, K.-C. (2011). A complementary electrochromic device based on Prussian blue and poly (ProDOT-Et2) with high contrast and high coloration efficiency. Solar Energy Materials and Solar Cells, 95(8), 2238–2245. https://doi.org/10.1016/j.solmat.2011.03.029
Qiu, M., Zhou, F., Sun, P., Chen, X., Zhao, C., & Mai, W. (2020). Unveiling the electrochromic mechanism of Prussian blue by electronic transition analysis. Nano Energy, 78, 105148. https://doi.org/10.1016/j.nanoen.2020.105148
Choi, D., Son, M., Im, T., Ahn, S.-H., & Lee, C. S. (2020). Crack-free fabrication of Prussian blue-based blending film for the dramatic enhancement of dual electrochromic device. Ceramics International, 46(13), 21008–21013. https://doi.org/10.1016/j.ceramint.2020.05.166
Choi, D., Kim, H.-J., Son, M., Kim, H.-S., Lee, H.-C., & Lee, C. S. (2019). Fabrication of a kinetically sprayed CuO ultra-thin film to evaluate CO gas sensing parameters. New Journal of Chemistry, 43(20), 7814–7821. https://doi.org/10.1039/c9nj00289h
Chun, D.-M., Kim, C.-S., Choi, J.-O., Lee, G.-Y., Lee, C. S., & Ahn, S.-H. (2014). Multilayer deposition of ceramic and metal at room temperature using nanoparticle deposition system (NPDS) and planarization process. The International Journal of Advanced Manufacturing Technology, 72(1), 41–46. https://doi.org/10.1007/s00170-013-5327-9
Jain, V., Yochum, H. M., Montazami, R., & Heflin, J. R. (2008). Millisecond switching in solid state electrochromic polymer devices fabricated from ionic self-assembled multilayers. Applied Physics Letters, 92(3), 033304. https://doi.org/10.1063/1.2834818
Jiao, Z., Wang, J., Ke, L., Liu, X., Demir, H. V., Yang, M. F., & Sun, X. W. (2012). Electrochromic properties of nanostructured tungsten trioxide (hydrate) films and their applications in a complementary electrochromic device. Electrochimica Acta, 63, 153–160. https://doi.org/10.1016/j.electacta.2011.12.069
Elgrishi, N., Rountree, K. J., McCarthy, B. D., Rountree, E. S., Eisenhart, T. T., & Dempsey, J. L. (2018). A practical beginner’s guide to cyclic voltammetry. Journal of Chemical Education, 95(2), 197–206. https://doi.org/10.1021/acs.jchemed.7b00361
Devan, R. S., Gao, S.-Y., Ho, W.-D., & Lin, J.-H. (2011). Electrochromic properties of large-area and high-density arrays of transparent one-dimensional β-Ta2O5 nanorods on indium-tin-oxide thin-films. Applied Physics Letters, 98(13), 133117. https://doi.org/10.1063/1.3568896
Kim, K., Choi, D., Kim, H., Lee, M., Chu, W., Ahn, S.-H., Chun, D.-M., & Lee, C. S. (2018). Investigation of varying particle sizes of dry-deposited WO3 particles in relation to performance of electrochromic cell. International Journal of Precision Engineering and Manufacturing-Green Technology, 5(3), 409–414. https://doi.org/10.1007/s40684-018-0043-4
Acknowledgements
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (2022R1F1A1071156) and by Korea Institute for Advancement of Technology(KIAT) grant funded by the Korea Government(MOTIE) (P0008425, The Competency Development Program for Industry Specialist).
Author information
Authors and Affiliations
Contributions
Dongwon Shin has done experiments and written the manuscript. Jiseon Kim has done experiments and discussed about the result data. Caroline Sunyong Lee has supervised the study related to the manuscript, proofread the paper. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shin, D., Kim, J. & Lee, C.S. Evaluation of V2O5 Film-Based Electrochromic Device with Dry-Deposited Ion Storage Layer. Int. J. Precis. Eng. Manuf. 24, 119–128 (2023). https://doi.org/10.1007/s12541-022-00731-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12541-022-00731-1