Abstract
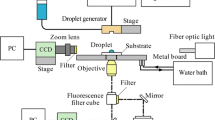
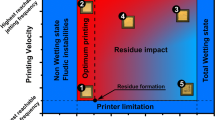
The deposit pattern of an evaporating picoliter droplet is studied changing the solvents (water, diethylene glycol, and formamide), mixing composition of the solvents and substrate temperature. When depinning occurs in the early stage of the evaporation period, concentrated solute (or partially deposited solute, if any) also retracts with the receding droplet, resulting in dome shaped deposit patterns. When pinning is maintained during most of the evaporation period, either a ring deposit or a relatively uniform deposit is obtained depending on the type of solvent and heating condition. These results are explained by interplay of (1) radial outward flow due to the strong evaporation flux at the contact line of a sessile droplet, (2) inward Marangoni flow due to evaporative cooling and solvent concentration gradient, and (3) solute mixing by diffusion in liquid.
Similar content being viewed by others
Abbreviations
- Csat :
-
saturated vapor concentration (kg/m3)
- Dsol :
-
solute diffusion coefficient in liquid (m2/s)
- Dvap :
-
vapor diffusion coefficient in air (cm2/s)
- d:
-
contact diameter (μm)
- hfg :
-
enthalpy of vaporization (kJ/kg)
- kB :
-
Boltzmann constant (J/K)
- L:
-
length scale (m)
- M:
-
molecular mass (g/mol)
- Psat :
-
saturation vapor pressure (kPa)
- R0 :
-
droplet radius before impact (μm)
- Rp :
-
radius of particle (μm)
- Tb :
-
boiling point at 1atm (°C)
- Ts :
-
substrate temperature (°C)
- tevap :
-
evaporation period (s)
- tsol,diff :
-
time scale for solute diffusion (s)
- tth,diff :
-
time scale for thermal diffusion (s)
- v:
-
droplet volume (pL)
- α:
-
thermal diffusivity of liquid (m2/s)
- θ:
-
contact angle (°)
- μ:
-
viscosity (mPa·s)
- ρ:
-
density (g/cm3)
- σ:
-
surface tension (mN/m)
- χ:
-
fraction of volatile solvent
References
Chung, J., Ko, S., Grigoropoulos, C. P., Bieri, N. R., Dockendorf, C., and Poulikakos, D., “Damage-free low temperature pulsed laser printing of gold nanoinks on polymers,” J. Heat Transfer, Vol. 127, No. 7, pp. 724–732, 2005.
Koo, H. S., Chen, M., and Pan, P. C., “Fabrication and Chromatic Characteristics of the Greenish LCD Colour-filter Layer with Nano-particle Ink using Inkjet Printing Technique,” Displays, Vol. 27, No. 3, pp. 124–129, 2006.
Deegan, R., Bakajin, O., and Duopont, T. F., “Capillary Flow as the Cause of Ring Stains from Dried Liquid Drops,” Nature, Vol. 389, pp. 827–829, 1997.
Hu, H. and Larson, R. G., “Analysis of the Microfluid Flow in an Evaporating Sessile Droplet,” Langmuir, Vol. 21, No. 9, pp. 3963–3971, 2005.
Tekin, E., de Gans, B. J., and Schubert, U. S., “Ink-jet Printing of Polymers — from Single Dots to Thin Film Libraries,” J. Mat. Chem., Vol. 14, No. 17, pp. 2627–2632, 2004.
de Gans, B. J. and Schubert, U. S., “Inkjet Printing of Welldefined Polymer Dots and Arrays,” Langmuir, Vol. 20, No. 18, pp. 7789–7793, 2004.
Kim, D., Jeong, S., Bong, B. K., and Moon, J., “Direct Writing of Silver Conductive Patterns: Improvement of Film Morphology and Conductance by Controlling Solvent Compositions,” App. Phy. Lett., Vol. 89, No. 26, Paper No. 264101, 2006.
Park, J. and Moon, J., “Control of Colloidal Particle Deposit Patterns within Picoliter Droplets Ejected by Ink-jet Printing,” Langmuir, Vol. 22, No. 8, pp. 3506–3513, 2006.
Sefiane, K., Tadrist, L., and Douglas, M., “Experimental Study of Evaporating Water-Ethanol Mixture Sessile Drop: Influence of Concentration,” Int. J. Heat and Mass Transfer, Vol. 46, No. 23, pp. 4527–4534, 2003.
Hu, H. R. and Larson, G., “Analysis of the Effects of Marangoni Stresses on the Microflow in an Evaporating Sessile Droplet,” Langmuir, Vol. 21, No. 9, pp. 3972–3980, 2005.
Hu, H. and Larson, R. G., “Marangoni Effect Reverses Coffeering Depositions,” J. Phy. Chem. B, Vol. 110, No. 14, pp. 7090–7094, 2006.
Lim, T., Jeong, J., Chung, J., and Chung, J. T., “Evaporation of Inkjet Printed Pico-liter Droplet on Heated Substrates with Different Thermal Conductivity,” J. Mech. Sci. Tech., Vol. 23, pp. 1788–1794, 2009.
Lim, T., Han, S., Chung, J., Chung, J. T., Ko, S., and Grigoropoulos, C. P., “Experimental Study on Spreading and Evaporation of Inkjet Printed Pico-liter Droplet on a Heated Substrate,” Int. J. Heat and Mass Transfer, Vol. 52, No. 1–2, pp. 431–441, 2009.
Lide, D. R., “CRC Handbook of Chemistry and Physics,” CRC Press, 2006.
Poling, B. E., Prausnitz, J. M., and O’Connell, J. P., “The Properties of Gases and Liquids,” McGraw-Hill, 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, T., Yang, J., Lee, S. et al. Deposit pattern of inkjet printed pico-liter droplet. Int. J. Precis. Eng. Manuf. 13, 827–833 (2012). https://doi.org/10.1007/s12541-012-0108-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12541-012-0108-1