Abstract
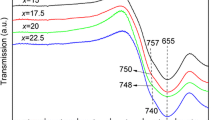
Tellurite glass frits with different SiO2/PbO ratios were produced and characterized for use in the Ag paste in the electrodes of polycrystalline Si solar cells. The thermophysical properties of the frits were determined using differential scanning calorimetry, and their fusion behaviors were studied using hot-stage microscopy. Glass frits in the Ag pastes reacted with the Si wafer. The interfacial structure between the Ag electrodes and the Si wafer was observed using scanning electrode microscopy. Depending on their compositions, the glass frits in the Ag paste exhibited different contact behaviors with the Si wafer substrate. This led to different interfacial structures between Ag electrodes and the Si wafer. The morphology of the interfacial structure significantly affected the electrical properties of the resulting Si solar cells. The viscosity of the fused glass frits at temperatures above their flow-point temperature affected the degree of Si wafer etching and the distribution of recrystallites on the n+ emitter.
Similar content being viewed by others
References
S. B. Rane, T. Seth, G. J. Phatak, and D. P. Amalnerkar, J. Electron. Mater. 15, 103 (2004).
M. Prudenziati, L. Moro, B. Morten, F. Sirotti, and L. Sardi, Act. Passive Electron. Compon. 13, 133 (1989).
S. Hwang, S. Lee, and H. Kim, J. Electroceram. 23, 351 (2009).
S. Y. Lee, S. H. Jin, S. M. Kim, and J. W. Kim, Met. Mater. Int. 20, 695 (2014).
K. Hong, S. Cho, J. Huh, H. J. Park, and J. W. Jeong, Met. Mater. Int. 15, 307 (2009).
Y. Zhang, Y. Yang, J. Zheng, W. Hua, and G. Chen, Mater. Chem. Phys. 114, 319 (2009).
Y. C. Shih, Y. H. Lin, J. P. You, and F. G. Shi, J. Electron. Mater. 42, 410 (2013).
Q. Che, H. Yang, L. Lu, and Y. Wang, Appl. Energy 112, 657 (2013).
D. Kim and H. Kim, J. Am. Ceram. Soc. 96, 774 (2013).
J. G. Bai, Z. Z. Zhang, J. N. Calata and G. Lu, IEEE Trans. Compon. Packag. Technol. 29, 589 (2006).
Y. Chiang, S. Wang, C. Lu, H. Lin, Lu. Wang, and Y. Ding, J. Electroceram. 31, 109 (2013).
M. Eberstein, H. F. Windisch, M. Peschel, J. Schilm, T. Seuthe, M. Wenzel, C. Kretzschmar, U. Partsch, Energy Proc. 27, 522 (2012).
S. Kim, S. Park, Y. D. Kim, H. Boo, H. Kim, S. Bae, H. Park, S. J. Tark, and D. Kim, Met. Mater. Int. 19, 1333 (2013).
M. B. Volf, Chemical Approach to Glass, pp.554–556, Elsevier, Amsterdam (1984).
A. Kaur, A. Khanna, C. Pesquera, F. González, and V. Sathe, J. Non-Cryst. Solids 356, 864 (2010).
B. V. R. Chowdari and P. P. Kumari, J. Non-Cryst. Solids 197, 31 (1996).
K. J. Hubbard and D. G. Schlom, J. Mater. Res 11, 2757 (1996).
M. J. Pascual, A. Durán, and M. O. Prado, Phys. Chem. Glasses 46, 512 (2005).
D. Ležal, J. Hoák, S. Karamazov, A. Skenár, and J. Navrátil, Phys. Chem. Glasses 42, 324 (2001).
H. Shim, S. Cho, H. Yie, and H. Kim, Ceram. Int. 41, 2196 (2015).
M. A. P. Silva, Y. Messaddeq, S. J. L. Ribeiro, M. Poulain, F. Villain, and V. Briois, J. Phys. Chem. Solids 62, 1055 (2001).
X. Pi, J. Chen, X. Cao, Z. Fu, L. Wang, and Q. Zhang, J. Synthetic Crystals 42, 1390 (2013).
B. Shanmugavelu and V. V. R. K. Kumar, J. Am. Ceram. Soc. 95, 2891 (2012).
H. R. Fernandes, D.U. Tulyaganov, A. Goel, M. J. Ribeiro, M. J. Pascual, and J. M. F. Ferreira, J. Eur. Ceram. Soc. 30, 2017 (2010).
N. Nagedra, U. Ramamurty, T. T. Goh, and Y. Li, Acta Mater. 48, 2063 (2000).
J. De, A. M. Umarji, and K. Chattopadhyay, Mater. Sci. Eng. A 449, 1062 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, S., Cho, S., Lee, J. et al. Reaction and interfacial structures between Ag paste with tellurite glass frits and Si wafer for solar cells. Met. Mater. Int. 21, 686–691 (2015). https://doi.org/10.1007/s12540-015-4567-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-015-4567-7