Abstract
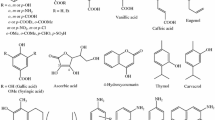
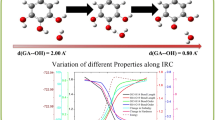
Health benefits of dietary phytochemicals have been suggested in recent years. Among 1000s of different compounds, Betalains, which occur in vegetables of the Cariophyllalae order (cactus pear fruits and red beet), have been considered because of reducing power and potential to affect redox-modulated cellular processes. The antioxidant power of Betalains is strictly due to the dissociation rate of the acid moieties present in all the molecules of this family of phytochemicals. Experimentally, only the pK a values of betanin were determined. Recently, it was evidenced it was evidenced as the acid dissociation, at different environmental pHs, affects on its electron-donating capacity, and further on its free radical scavenging power. The identical correlation was studied on another Betalains family compound, Betalamic Acid. Experimental evidences showed that the free radical scavenging capacity of this compound drastically decreases at pH > 5, but pK a values were experimentally not measured. With the aim to justify the Betalamic Acid behavior as free radical scavenger, in this paper we tried to predict in silico the pK a values by means different approaches. Starting from the known experimental pK as of acid compounds, both phytochemicals and small organic, two empirical approaches and quantum–mechanical calculation were compared to give reliable prediction of the pK as of Betalamic Acid. Results by means these computational approaches are consistent with the experimental evidences. As shown herein, in silico, the totally dissociated species, at the experimental pH > 5 in solution, is predominant, exploiting the higher electron-donating capability (HOMO energy). Therefore, the computational estimated pK a values of Betalamic Acid resulted very reliable.







Similar content being viewed by others
References
Carratù B, Sanzini E (2005) Sostanze biologicamente attive presenti negli alimenti di origine vegetale. Ann Ist Super Sanità 41:7–16 (in Italian)
Ronco A, De Stefani E, Boffetta P, Deneo-Pellegrini H, Mendilaharsu M, Leborgne F (1999) Vegetables, fruits, and related nutrients and risk of breast cancer, a case–control study in Uruguay. Nutr Cancer 35:111–119
Agarwal S, Rao AV (2000) Tomato lycopene and its role in human health and chronic diseases. Can Med Assoc J 163:739–744
McKeown N (1999) Antioxidants and breast cancer. Nutr Rev 57:321–323
Scalbert A, Johnson IT, Saltmarsh M (2005) Polyphenols, antioxidants and beyond. Am J Clin Nutr 81:215S–217S
Raederstorff D (2009) Antioxidant activity of olive polyphenols in humans, a review. Int J Vitam Nutr Res 79:152–165
Herr I, Büchler MW (2010) Dietary constituents of broccoli and other cruciferous vegetables, implications for prevention and therapy of cancer. Cancer Treat Rev 36:377–383
Tesoriere L, Allegra M, Butera D, Livrea MA (2004) Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs, potential health effects of betalains in humans. Am J Clin Nutr 80:941–945
Frank T, Stintzing FC, Carle R, Bitsch I, Quaas D, Straß G, Bitsch R, Netzel M (2005) Urinary pharmacokinetics of betalains following consumption of red beet juice in healthy humans. Pharmacol Res 52:290–297
Kanner J, Harel S, Granit R (2001) Betalains a new class of dietary cationized antioxidants. J Agric Food Chem 49:5178–5185
Holt RR, Lazarus SA, Sullards MC, Zhu QY, Schramm DD, Hammerstone JF, Fraga CG, Schmitz HH, Keen CL (2002) Procyanidin dimer B2 [epicatechin-4β-8-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am J Clin Nutr 76:798–804
Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M (2006) (–)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 103:1024–1029
Tesoriere L, Fazzari M, Angileri F, Gentile C, Livrea MA (2008) In vitro digestion of betalainic foods. Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. J Agric Food Chem 56:10487–10492
Gliszczyńska-Swigło A, Szymusiak H, Malinowska P (2006) Betanin, the main pigment of red beet, molecular origin of its exceptionally high free radical-scavenging activity. Food Addit Contam 23:1079–1087
Tesoriere L, Gentile C, Angileri F, Attanzio A, Tutone M, Allegra M, Livrea MA (2013) Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. Eur J Nutr 52:1077–1087
Gandía-Herrero F, Escribano J, García-Carmona F (2012) Purification and antiradical properties of the structural unit of betalains. J Nat Prod 75:1030–1036
Fraczkiewicz R (2007) In: Testa B, van de Waterbeemd H (eds) Comprehensive medicinal chemistry II, vol 5. Elsevier, pp 603–626
Perrin DD (1965) Dissociation constants of organic bases in aqueous solution: supplement 1972. Butterworths, London
Serjeant E, Dempesey BL (1979) Ionization constant of organic acids in aqueous solution. Pergamon, Oxford
Albert A (1963) In: Katritzky AR (ed) Ionization constant of heterocyclic substances in physical methods in heterocyclic chemistry. chapter 5, Academic Press, New York
Sober HA (1968) CRC handbook of biochemistry. CRC Press, Cleveland
Perrin DD, Dempsey B, Serjeant EP (1981) pKa prediction for organic acids and bases. Chapman & Hall, London
Dawson RMC, Elliott DC, Elliott WH, Jones KM (1986) Data for biochemical research. Oxford Science, Oxford
Washburn EW (1929) International critical tables. McGraw-Hill, New York
Hasanain F, Wang ZY (2008) New one-step synthesis of polyimides in salicylic acid. Polymer 49:831–835
Borges F, Lima JLFC, Pinto I, Reis S, Siquet C (2003) Application of a potentiometric system with data-analysis computer programs to the quantification of metal-chelating activity of two natural antioxidants, caffeic acid and ferulic acid. Helv Chim Acta 86:3081–3087
Abbasi S, Daneshfar A, Hamdghadare S, Farmany A (2011) Quantification of sub-nanomolar levels of gallic acid by adsorptive stripping voltammetry. Int J Electrochem Sci 6:4843–4852
Beltrán JL, Sanli N, Fonrodona G, Barrón D, Özkan G, Barbosa J (2003) Spectrophotometric, potentiometric and chromatographic pKa values of polyphenolic acids in water and acetonitrile–water media. Anal Chim Acta 484:253–264
Du H, Chen X (2009) CD-MEKC method to analyze triterpene acids in traditional chinese medicines. J Braz Chem Soc 20:1268–1274
Wybraniec S (2005) Formation of decarboxylated betacyanins in heated purified betacyanin fractions from red beet root Beta vulgaris L. monitored by LC–MS/MS. J Agric Food Chem 53:3483–3487
Szegezdi J, Csizmadia F (2004) Prediction of dissociation constant using microconstants. In: 27th ACS American Chemical Society national meeting, Anaheim, CA, 28 March–1 April
Szegezdi J, Csizmadia F (2007) Method for calculating pK a values of small and large molecules. In: 233rd ACS American Chemical Society national meeting, Chicago, IL, 25–29 March
Epik version 2.0 (2009) Schrödinger, LLC, New York
Shelley JC, Cholleti A, Frye LL, Greenwood JR, Timlin MR, Uchimaya M (2007) Epik, a software program for pKa prediction and protonation state generation for drug-like molecules. J Comput Aided Mol Des 21:681–691
Bochevarov AD, Harder E, Hughes TF, Greenwood JR, Braden DA, Philipp DM, Rinaldo D, Halls MD, Zhang J, Friesner RA (2013) Jaguar, a high-performance quantum chemistry software program with strengths in life and materials sciences. Int J Quantum Chem 113:2110–2142
Jaguar version 7.6 (2009) Schrödinger, LLC, New York
MacroModel, version 10.0 (2013) Schrödinger, LLC, New York
Liao C, Nicklaus MC (2009) Comparison of nine programs predicting pKa values of pharmaceutical substances. J Chem Inf Model 4912:2801–2812
Neumeyer K, Ross T, Thomson G, McMeekin TA (1997) Validation of a model describing the effects of temperature and water activity on the growth of psychrotrophic pseudomonads. Int J Food Microbiol 38:55–63
Mellefont LA, McMeekin TA, Ross T (2003) Performance evaluation of a model describing the effects of temperature, water activity, pH and lactic acid concentration on the growth of Escherichia coli. Int J Food Microbiol 82:45–58
Nilsson T (1970) Studies into the pigmentsin beetroot Beta vulgaris L. vulgaris var. rubra L. Lantbrukshoegskolans Annaler 36:179–219
Manchester J, Walkup G, Rivin O, You Z (2010) Evaluation of pKa estimation methods on 211 druglike compounds. J Chem Inf Model 50:565–571
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tutone, M., Lauria, A. & Almerico, A.M. Theoretical Determination of the pK a Values of Betalamic Acid Related to the Free Radical Scavenger Capacity: Comparison Between Empirical and Quantum Chemical Methods. Interdiscip Sci Comput Life Sci 8, 177–185 (2016). https://doi.org/10.1007/s12539-015-0101-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-015-0101-3