Abstract
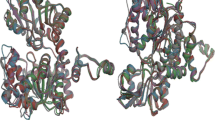
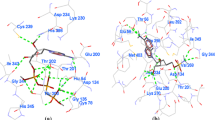
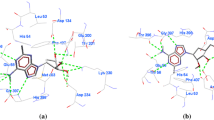
The Plasmodium falciparum S-adenosyl-L-homocysteine hydrolase (pfSAHH) enzyme has been considered as a potential chemotherapeutic target against malaria due to the amino acid differences found on binding sites of pfSAHH related to human SAHH. It has been reported that noraristeromycin and some curcumin derivatives have potential binding with the largest cavity of pfSAHH, which is also related to the binding with Nicotinamide-Adenine-Dinucleotide (NAD) and Adenosine (ADN). Our present work focuses on docking and ADMET studies to select potential inhibitors of pfSAHH. The binding of the selected inhibitor of the PfSAHH active site was analyzed using Molegro Virtual Docker. In this study, curcumin and its derivatives have been found to have higher binding affinity with pfSAHH than noraristeromycin. Seven amino acid residues Leu53, His54, Thr56, Lys230, Gly397, His398 and Phe407 of pfSAHH involved in binding with curcumin, are the same as those for noraristeromycin, which reveals that curcumin and noraristeromycin bind in the same region of pfSAHH. Curcumin has shown a strong interaction with hydrophobic amino acid residues of pfSAHH. Molecular Docking and ADMET predictions suggest that curcumin can be a potent inhibitor of pfSAHH with ability to modulate the target in comparatively smaller dose. Therefore, curcumin is likely to become a good lead molecule for the development of effective drug against malaria.
Similar content being viewed by others
References
Ando, T., Kojima, K., Chahota, P., Kozaki, A., Milind, N.D., Kitade, Y. 2008. Synthesis of 4′-modified noraristeromycins to clarify the effect of the 4′-hydroxyl groups for inhibitory activity against Sadenosyl-l-homocysteine hydrolase. Bioorg Med Chem Lett 18, 2615–2618.
Brady, R.L., Cameron, A. 2004. Structure-based approaches to the development of novel anti-malarials. Curr Drug Targets, 137–149.
Efferth, T., Herrmann, F., Tahrani, A., Wink, M. 2011. Cytotoxic activity of secondary metabolites derived from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine 18, 959–969.
Ertl, P., Rohde, B., Selzer, P. 2000. Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties. J Med Chem 43, 3714–3417.
Garah, F.B., Stigliani, J.L., Cosldan, F., Meunier, B., Robert, A. 2009. Docking studies of structurally diverse antimalarial drugs targeting PfATP6: No correlation between in silico binding affinity and in vitro antimalarial activity. ChemMedChem 4, 1469–1479.
Ji, H.F., Shen, L. 2009. Interactions of curcumin with the PfATP6 model and the implications for its antimalarial mechanism. Bioorg Med Chem Lett 19, 2453–2455.
Jorgensen, W.L. 1998. BOSS — Biochemical and organic simulation system. In: Schleyer, P.V.R. (Ed.) The Encyclopedia of Computational Chemistry, John Wiley & Sons Ltd., Athens, USA, 3281–3285.
Jung, M., Kim, H., Nam, K.Y., No, K.T. 2005. Threedimensional structure of Plasmodium falciparum Ca2+-ATPase (PfATP6) and docking of artemisinin derivatives to PfATP6. Bioorg Med Chem Lett 15, 2994–2997.
Kalani, K., Yadav, D.K., Khan, F., Srivastava, S.K., Suri, N. 2011. Pharmacophore, QSAR and ADME based semi-synthesis and in-vitro evaluation of ursolic acid analogs for anti-cancer activity. J Mol Model 8, 3389–3413.
Kitade, Y., Kozaki, A., Miwa, T., Nakanishi, M., Yatome, C. 2000. Synthesis of carbocyclic nucleosides and their SAH hydrolase inhibitory activities. Nucleic Acids Symp Ser 44, 111–112.
Kitade, Y., Kojima, H., Zulfiqur, F., Kim, H.S., Wataya, Y. 2003a. Synthesis of 2-fluoronoraristeromycin and its inhibitory activity against Plasmodium falciparum S-adenosyl-L-homocysteine hydrolase. Bioorg Med Chem Lett 13, 3963–3965.
Kitade, Y., Kojima, H., Zulfiqur, F., Yabe, S., Yamagiwa, D., Ito, Y., Nakanishi, M., Ueno, Y., Kim, H.S., Wataya, Y. 2003b. Synthesis of carbocyclic and acyclic nucleosides possessing 2-fluoroadenine derivatives and their inhibitory activities against Plasmodium falciparum SAH hydrolase. Nucl Acid Res (Suppl. 3), 5–6.
Kojima, H., Yamaguchi, T., Kozaki, A., Nakanishi, M., Ueno, Y., Kitade, Y. 2002. Synthesis of noraristeromycin analogues possessing SAH hydrolase inhibitory activity for the development of antimalaria agents. Nucl Acid Res (Suppl. 2), 141–142.
Lipinski, C.A., Lombardo, F., Dominy, B.W., Fenney, P.J. 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46, 3–26.
Lowe, R., Glen, R., Mitchell, J.B.O. 2010. Predicting phospholipidosis using machine learning. Mol Pharm 7, 1708–1714.
Matthews, E.J., Kruhlak, N.L., Benz, R.D., Contrera, J.F. 2004a. Assessment of the health effects of chemicals in humans: I. QSAR estimation of the maximum recommended therapeutic dose (MRTD) and no effect level (NOEL) of organic chemicals based on clinical trial data. Curr Drug Disc Tech 1, 61–76.
Matthews, E.J., Kruhlak, N.L., Weaver, J.L., Benz, R.D., Contrera, J.F. 2004b. Assessment of the health effects of chemicals in humans: II. Construction of an adverse effects database for QSAR modeling. Curr Drug Disc Tech 1, 243–254.
Meena, A., Yadav, D.K., Srivastava, A., Khan, F., Chanda, D, Chattopadhyay, S.K. 2011. In silico exploration of anti-inflammatory activity of natural Coumarinolignoids. Chem Biol Drug Des 78, 567–579.
Mimche, P.N., Taramelli, D., Vivas, L. 2011. The plant-based immunomodulator curcumin as a potential candidate for the development of an adjunctive therapy for cerebral malaria. Malar J 15, 10Suppl 1, S10.
Mithani, S.D., Bakatselou, V., TenHoor, C.N., Dressman, J.B. 1996. Estimation of the increase in solubility of drugs as a function of bile salt concentration. Pharm Res 13, 163–167.
Naik, P.K., Srivastava, M., Bajaj, P., Jain, S., Dubey, A., Ranjan, P., Kumar, R., Singh, H. 2011. The binding modes and binding affinities of artemisinin derivatives with Plasmodium falciparum Ca2+-ATPase (PfATP6). J Mol Model 17, 333–357.
Nakanishi, M., Yabe, S., Tanaka, N., Ito, Y., Nakamura, K.T., Kitade, Y. 2005. Mutational analyses of Plasmodium falciparum and human Sadenosylhomocysteine hydrolases. Mol Biochem Parasitol 143, 146–151.
Padmanaban, G., Nagaraj, V.A., Rangarajan, P.N. 2007. Drugs and drug targets against malaria. Curr Sci 92, 1545–1555.
Pajeva, I.K., Globisch, C., Wiese, M. 2009. Combined pharmacophore modeling, docking, and 3D QSAR studies of ABCB1 and ABCC1 transporter inhibitors. Chem Med Chem 4, 1883–1896.
Rasoanaivo, P., Wright, C.W., Willcox, M.L., Gilbert, B. 2011. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar J 15,10 Suppl 1, S4.
Reddy, R.C., Vatsala, P.G., Keshamouni, V.G., Padmanaban, G., Rangarajan, P.N. 2005. Curcumin for malaria therapy. Biochem Biophys Res Commun 326, 472–474.
Sadowski, J., Gasteiger, J., Klebe, G. 1994. Comparison of automatic three-dimensional model builders using 639 X-ray structures. J Chem Inf Comput Sci 34, 1000–1008.
Sanguinetti, M.C., Tristani-Firouzi, M. 2006. hERG potassium channels and cardiac arrhythmia. Nature 440, 463–469.
Sharma, S.K., Kapoor, M., Ramya, T.N., Kumar, S., Kumar, G., Modak, R., Sharma, S., Surolia, N., Surolia, A. 2003. Identification, characterization, and inhibition of Plasmodium falciparum beta-hydroxyacylacyl carrier protein dehydratase (FabZ). J Biol Chem 278, 45661–45671.
Shi, W., Ting, L.M., Kicska, G.A., Lewandowicz, A., Tyler, P.C., Evans, G.B., Furneaux, R.H., Kim, K., Almo, S.C., Schramm, V.L. 2004. Plasmodium falciparum purine nucleoside phosphorylase:crystal structures, immucillin inhibitors, and dual catalytic function. J Biol Chem 279, 18103–18106.
Singh, D.V., Agarwal, S., Kesharwani, R.K., Misra, K. 2012. Molecular modeling and computational simulation of the photosystem-II reaction center to address isoproturon resistance in Phalaris minor. J Mol Model 18, 3903–3913.
Singh, D.B., Gupta, M.K., Kesharwani, R.K., Misra, K. 2013. Comparative docking and ADMET study of some curcumin derivatives and herbal congeners targeting β-amyloid. Netw Model Anal Health Inform Bioinforma 2, 13–27.
Tagboto, S., Townson, S. 2001. Antiparasitic properties of medicinal plants and other naturally occurring products. Adv Parasitol 50, 199–295.
Tanaka, N., Nakanishi, M., Kusakabe, Y., Shiraiwa, K., Yabe, S., Ito, Y., Kitade, Y., Nakamura, K.T. 2004. Crystal structure of S-adenosyl-L-homocysteine hydrolase from the human malaria parasite Plasmodium falciparum. J Mol Biol 343, 1007–1017.
Thomsen, R., Christensen, M.H. 2006. MolDock: A new technique for high-accuracy molecular docking. J Med Chem 49, 3315–3321.
Tschan, S., Mordmller, B., Kun, J.F. 2011. Threonine peptidases as drug targets against malaria. Expert Opin Ther Targets, 365–378.
Wang, J. 2009. Comprehensive assessment of ADME risks in drug discovery. Curr Pharm 15, 2195–2219.
Yadav, D.K., Khan, F., Negi, A.S. 2011. Pharmacophore modeling, molecular docking, QSAR, and in silico ADMET studies of gallic acid derivatives for immunomodulatory activity. J Mol Model 18, 2513–2525.
Yadav, D.K., Meena, A., Srivastava, A., Chanda, D., Khan, F., Chattopadhyay, S.K. 2010. Development of QSAR model for immunomodulatory activity of natural Coumarinolignoids. Drug Design, Development & Therapy 4, 173–186.
Yang, J., Chen, C. 2004. GEMDOCK: A generic evolutionary method for molecular docking. Proteins 55, 288–304.
Yang, X., Hu, Y., Yin, D.H., Turner, M.A., Wang, M., Borchardt, R.T., Howell, P.L., Kuczera, K., Schowen, R.L. 2003. Catalytic strategy of S-adenosyl-L-homocysteine hydrolase: Transition-state stabilization and the avoidance of abortive reactions. Biochemistry 42, 1900–1909.
Yuan, C.S., Saso, Y., Lazarides, E., Borchardt, R.T., Robins, M.J. 1999. Recent advances in S-adenosyl-L-homocysteine hydrolase inhibitors and their potential clinical applications. Expert Opin Ther Patents 9, 1197–1206.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, D.B., Gupta, M.K., Singh, D.V. et al. Docking and in silico ADMET studies of noraristeromycin, curcumin and its derivatives with Plasmodium falciparum SAH hydrolase: A molecular drug target against malaria. Interdiscip Sci Comput Life Sci 5, 1–12 (2013). https://doi.org/10.1007/s12539-013-0147-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-013-0147-z