Abstract
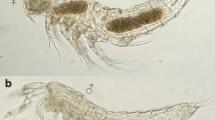
The new species, Paroctopus cthulu sp. nov. Leite, Lima, Lima and Haimovici was recorded from shallow coastal waters of south and southeastern Brazil, where most specimens were found sheltered in marine debris. It is a small octopus; adults are less than 35 mm mantle length (ML) and weight around 15 g. It has short- to medium-sized arms, enlarged suckers on the arms of both males and females, a relatively large beak (9% ML) and medium to large mature eggs (3.5 to > 9 mm). The characteristics of hatchlings of two brooding females, some of their anatomical features, and in situ observations of their behavior are a clue to the life history of it and closely related pygmy octopuses. The Bayesian phylogenetic analysis showed that Paroctopus cthulu sp. nov. is grouped in a well-supported clade of Paroctopus Naef, 1923 species, clearly distinct from Octopus joubini Robson, 1929 and Paroctopus mercatoris (Adam, 1937) from the Northwestern Atlantic. The description of this new species, living in habitat altered by humans, debris in shallow water off Brazil, offered an opportunity not only to evaluate the relationship among the small octopuses of the western Atlantic, Caribbean and eastern Pacific, but also their adaptation to the Anthropocene period.










Similar content being viewed by others
References
Acosta-Jofré MS, Sahade R, Laudien J, Chiappero MB (2012) A contribution to the understanding of phylogenetic relationships among species of the genus Octopus (Octopodidae: Cephalopoda). Sci Mar 76:311–318. https://doi.org/10.3989/scimar.03365.03B
Adam W (1936) Notes sur les Céphalopodes, VI: Une nouvelle espécie d´Octopus (Octopus hummelinck sp. nov.) des Indes occidentales Néerlandaises. Bull Du Musée Royal D´historie Naturelle De Belgique 12(40):1–3
Adam W (1937) Resultats scientifiques des croiseres du Navire–école Belge “Mercator.” Mémoires Du Musée Royal D´histoire Naturelle De Belgique 9(2):43–82
Adam W (1941) Notes sur les Cephalopodes. XV. Sur la valeur diagnostique de la radule chez les Cephalopodes Octopodes. Bull Mus Roy Hist Nat Belg 17(38):1–19
Albertin CB, Simakov O, Mitros T, Yan Wang Z, Pungor JR, Edsinger-Gonzales E, Brenner S, Ragsdale CW, Rokhsar DS (2015) The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature 524:220–222. https://doi.org/10.1038/nature14668
Alves MS, Silva MA, Melo Júnior M, Paranaguá MN, Pinto SL (2006) Zooartesanato comercializado em Recife, Pernambuco, Brasil. Rev Brasil Zoosci 8:99–109
Amor MD, Norman MD, Cameron HE, Strugnell JM (2014) Allopatric speciation within a cryptic species complex of Australasian octopuses. PLoS ONE 9:1–6. https://doi.org/10.1371/journal.pone.0098982
Anderson RC, Hughes PD, Mather JA, Steele CW (1999) Determination of the diet of Octopus rubescens Berry, 1953 (Cephalopoda: Octopodidae), through examination of its beer bottle dens in Puget Sound. Malacol 4:455–460
Arocha F, Urosa LJ (1982) Cefalópodos del género Octopus en el área nororiental de Venezuela. Bol Inst Ocean Venezuela Univers Orient 2:167–189
Avendaño O, Roura A, Cedillo–Robles CE, González AF, Rodríguez–Canul R, Velázquez–Abunader I, Guerra A (2020) Octopus americanus: a cryptic species of the O. vulgaris species complex redescribed from the Caribbean. Aquat Ecol 54:909–925. https://doi.org/10.1007/s10452-020-09778-6
Barros F, Santos D, Reis A, Martins A, Dodonov PJ, Nunes JACC (2020) Choosing trash instead of nature: Sea urchin covering behavior. Mar Poll Bull 155:111188. https://doi.org/10.1016/j.marpolbul.2020.111188
Berry SS (1953) Preliminary diagnoses of six west American species of Octopus. Leaf Malacol 1(10):51–58
Boletzky SV (1974) The “larvae” of Cephalopoda: a review. Thalass Jugo 10:45–76
Boletzky SV, Boletzky MVV (1969) First results in rearing Octopus joubini Robson, 1929. Institute of Marine Sciences, University of Miami 1056:56–61
Boletzky SV, Fuentes M, Offner N (2002) Developmental features of Octopus macropus Rissso, 1826 (Mollusca, Cephalopoda). Vie Et Milieu 52:209–216
Braid HE, Bolstad KSR (2019) Cephalopod biodiversity of the Kermadec Islands: implications for conservation and some future taxonomic priorities. Invertebr Syst 33:402–425. https://doi.org/10.1071/IS18041
Brunel P, Besner M, Messier D, Poirier L, Granger D, Weinstein M (1978) Le traîneau suprabenthique MACER-GIROQ: appareil amélioré pour l’échantillonnage quantitatif étagé de la petite faune nageuse an voisinage du fond The MACER–GIROQ suprabenthic sled: an improved device for quantitative two level sampling of the small swimming fauna near the bottom. Int Rev Gesam Hydrobiol Hydrograp 63:815–829. https://doi.org/10.1002/iroh.19780630612
Burgess LA (1966) A study of the morphology and biology of Octopus hummelincki Adam, 1936 (Mollusca: Cephalopoda). Bull Mar Sci 16:762–813
Carlini DB, Young RE, Vecchione M (2001) A molecular phylogeny of the Octopoda (Mollusca: Cephalopoda) evaluated in light of morphological evidence. Mol Phylogenet Evol 21:388–397. https://doi.org/10.1006/MPEV.2001.1022
Cigliano JA (1995) Assessment of the mating history of female pygmy octopuses and a possible sperm competition mechanism. Anim Behav 49:849–851. https://doi.org/10.1016/0003-3472(95)90060-8
Dai L, Zheng X, Kong L, Li Q (2012) DNA barcoding analysis of Coleoidea (Mollusca: Cephalopoda) from Chinese waters. Mol Ecol Resour 12:437–447. https://doi.org/10.1111/j.1755-0998.2012.03118.x
deLuna Sales JBL, Haimovici M, Ready JS, Souza RF, Ferreira Y, Pinon JCS, Costa LFC, Asp NE, Sampaio I, Schneider H (2019) Surveying cephalopod diversity of the Amazon reef system using samples from red snapper stomachs and description of a new genus and species of octopus. Sci Rep 9(5956):1–16. https://doi.org/10.1038/s41598-019-42464-8
Deryckere A, Styfnals R, Vidal EAG, Almansa E, Seuntjens E (2020) A practical staging atlas to study embryonic development of Octopus vulgaris under controlled laboratory conditions. BMC Dev Biol 20:7. https://doi.org/10.1186/s12861-020-00212-6
Díaz–Santana–Iturrios M, Salinas–Zavala CA, García–Rodríguez FJ, Granados–Amores J (2019) Taxonomic assessment of species of the genus Octopus from the northeastern Pacific via morphological, molecular and morphometric analyses. PeerJ 7:e8118. https://doi.org/10.7717/peerj.8118
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973. https://doi.org/10.1093/molbev/mss075
Eidemiller JA (1972) Significant associations of the motile epibenthos of the turtle–grass beds of St. Joseph Bay, Florida. M.S. Thesis, Florida State University
Férussac AE, d’Orbigny A (1835) 1835–1848 Histoire naturelle générale et particulière céphalopodes acétabulifères vivants et fossiles. Paris: J.B. Balliere. https://doi.org/10.5962/bhl.title.156830
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotech 3:294–299
Forsythe JW (1984) Octopus joubini (Mollusca: Cephalopoda): a detailed study of growth through the full life cycle in a closed seawater system. J Zool 202:393–417. https://doi.org/10.1111/j.1469-7998.1984.tb05091.x
Forsythe JW, Hanlon RT (1980) A closed marine culture system for rearing Octopus joubini and other large–egged benthic octopods. Lab Anim 14:137–142. https://doi.org/10.1258/002367780780942737
Forsythe JW, Toll RB (1991) Clarification of the western Atlantic Ocean pygmy octopus complex: the identity and life history of Octopus joubini (Cephalopoda: Octopodidae). Bull Mar Sci 49:88–97
Gebhardt K, Knebelsberger T (2015) Identification of cephalopod species from the North and Baltic Seas using morphology, COI and 18S rDNA sequences. Helgol Mar Res 69:259–271. https://doi.org/10.1007/s10152-015-0434-7
Gleadall IG (2013) A molecular sequence proxy for Muusoctopus januarii and calibration of recent divergence among a group of mesobenthic octopuses. J Exp Mar Ecol 447:106–122. https://doi.org/10.1016/J.JEMBE.2013.02.017
Gould AA (1852) Mollusca and shells. In: United States exploring expedition during the years 1838, 1839, 1840, 1841, 1842 under the command of Charles Wilkes. Boston: Gould & Lincoln, pp 475–476. https://doi.org/10.5962/bhl.title.61454
Grimpe G (1925) Zur kenntnis der Cephalopoden fauna der Nordsee. Wiss Meeresunter Abteil Helg Neue Folge 16:1–124
Haimovici M (1985) Class Cephalopoda. In: Rios EC (ed) Seashells of Brazil. Rio Grande: FURG, pp 183–288
Haimovici M, Santos RA, Fischer LG (2009) Class Cephalopoda. In: Rios EC (ed) Compendium of Brazilian Sea Shells. Rio Grande: Evangraf, pp 610–649
Hanlon RT, Hixon RF (1980) Body patterning and field observations of Octopus burryi Voss. Bull Mar Sci 30(4):749–755
Hanlon RT (1983) Octopus joubini. In: Boyle PR (ed) Cephalopod life cycles, vol 1. Academic Press, London, pp 293–310
Hanlon RT (1988) Behavioral and body patterning characters useful in taxonomy and field identification of cephalopods. Malacol 29(1):247–264
Haraway D (2015) Anthropocene, capitalocene, plantationocene, chthulucene: making kin. Environ Human 6:159–165. https://doi.org/10.1215/22011919-3615934
Hochberg FG, Nixon M, Toll RB (1992) Octopoda. In: Sweeney MJ, Roper CFE, Mangold KM, Clarke MR, Boletzky SV (eds) “Larval” and juvenile cephalopods: a manual for their identification. Washington: Smithson Contributions to Zoology, pp 213–280
Hochner B, Shomrat T, Fiorito G (2006) The octopus: a model for a comparative analysis of the evolution of learning and memory mechanisms. Biol Bull 210:308–317. https://doi.org/10.2307/4134567
Hoorn C (1994) An environmental reconstruction of the palaeo–Amazon River system (Middle–Late Miocene, NW Amazonia). Palaeogeog, Palaeoclim Palaeoecol 112:187–238. https://doi.org/10.1016/0031-0182(94)90074-4
Huffard C, Hochberg FG (2005) Description of a new species of the genus Amphioctopus (Mollusca: Octopodidae) from the Hawaiian Islands. Mol Res 25:113–128
Huffard CL, Saarman N, Hamilton H, Simison WB (2010) The evolution of conspicuous facultative mimicry in octopuses: an example of secondary adaptation? Biol J Linn Soc 101:68–77. https://doi.org/10.1111/j.1095-8312.2010.01484.x
Iribarne OO (1990) Use of shelter by the small Patagonian octopus Octopus tehuelchus: availability, selection and effects on fecundity. Mar Ecol Prog Ser 66:251–258. https://doi.org/10.3354/meps066251
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL (2015) Plastic waste inputs from land into the ocean. Science 347:768–771. https://doi.org/10.1126/science.1260352
Jereb P, Roper CFE, Norman MD, Finn JK (2014) Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Vol 3. Octopods and Vampire squids. Rome: Food and Agriculture Organization of the United Nations
Jesus MD, Sales JBL, Martins RS, Ready JS, Costa TAS, Ablett JD, Schiavetti A (2021) Traditional knowledge aids description when resolving the taxonomic status of unsettled species using classical and molecular taxonomy: The case of the shallow-water octopus Callistoctopus furvus (Gould, 1852) from the western Atlantic Ocean. Front Mar Sci 7. https://doi.org/10.3389/fmars.2020.595244
Kaneko N, Kubodera T, Iguchis K (2011) Taxonomic study of shallow–water octopuses (Cephalopoda: Octopodidae) in Japan and adjacent waters using mitochondrial genes with perspectives on octopus DNA barcoding. Malacologia 54:97–108. https://doi.org/10.4002/040.054.0102
Katsanevakis S, Verriopoulos G (2004) Den ecology of Octopus vulgaris Cuvier, 1797, on soft sediment: availability and types of shelter. Sci Mar 68:147–157. https://doi.org/10.3989/scimar.2004.68n1147
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kowalewski M, Domènech R, Martinell J (2014) Vanishing clams on an Iberian beach: local consequences and global implications of accelerating loss of shells to tourism. PLoS ONE 9(1):e83615. https://doi.org/10.1371/journal.pone.0083615
Leite TS, Haimovici M (2006) Presente conhecimento da biodiversidade e habitat dos polvos (Cephalopoda: família Octopodidae) de águas rasas das ilhas oceânicas do Nordeste Brasileiro. In: Alves RJV, Castro JWA (ed) Ilhas Oceânicas Brasileiras – da Pesquisa ao Manejo. Brasília: MMA, pp 199–214
Leite TS, Haimovici M, Molina W, Warnke K (2008) Morphological and genetic description of Octopus insularis, a new cryptic species in the Octopus vulgaris complex (Cephalopoda: Octopodidae) from the tropical southwestern Atlantic. J Moll Stud 74:63–74. https://doi.org/10.1093/mollus/eym050
Lima FD, Berbel-Filho WM, Leite TS, Rosas C, Lima SMQ (2017) Occurrence of Octopus insularis Leite and Haimovici, 2008, in the tropical northwestern Atlantic and implications of species misidentification to octopus fisheries management. Mar Biodiv 47:723–734. https://doi.org/10.1007/s12526-017-0638-y
Lima FD, Strugnell JM, Leite TS, Lima SMQ (2020) A biogeographic framework of octopod species diversification: the role of the Isthmus of Panama. Peer J 2020:1–19. https://doi.org/10.7717/peerj.8691
Lovecraft HP (1984) The Call of Cthulhu. In: Joshi ST (ed) The Dunwich Horror and Others. 9th ed. Sauk City: Arkham House
Magallón-Gayón E, del Río-Portilla MÁ, de los Angeles Barriga-Sosa I, (2019) The complete mitochondrial genomes of two octopods of the eastern Pacific Ocean: Octopus mimus and “Octopus” fitchi (Cephalopoda: Octopodidae) and their phylogenetic position within Octopoda. Mol Biol Rep 47:943–952. https://doi.org/10.1007/s11033-019-05186-8
Mangold K (1998) The Octopodidae from the eastern Atlantic Ocean and the Mediterranean sea. In: Voss NA, Vecchione M, Toll RB, Sweeney MJ (eds) Systematics and Biogeography of Cephalopods. Washington: Smithsonian Contribution to Zoology, pp 521–528
Mather JA (1972) Preliminary observations on the behaviour of Octopus joubini Robson, 1929. Dissertation, Florida State University
Mather JA (1978) Mating behavior of Octopus joubini Robson. Veliger 21:265–267
Mather JA (1980a) Some aspects of food intake in Octopus joubini Robson. Veliger 22:286–290
Mather JA (1980b) Social organization and use of space by Octopus joubini in a semi–natural situation. Bull Mar Sci 30:848–857
Mather J (1982a) Choice and competition: their effects on occupancy of shell homes by Octopus joubini. Mar Behav Physiol 8:285–293. https://doi.org/10.1080/10236248209387025
Mather JA (1982b) Factors affecting the spatial distribution of natural populations of Octopus joubini Robson. Anim Behav 30:1166–1170. https://doi.org/10.1016/S0003-3472(82)80207-8
Mather JA (1984) Development of behaviour in Octopus joubini Robson, 1929. Vie Et Milleu 34:17–20
McLean R (1983) Gastropod shells: A dynamic resource that helps shape benthic community structure. J Exp Mar Biol Ecol 69:151–174. https://doi.org/10.1016/0022-0981(83)90065-5
Monfort D (1802) Histoire naturelle, générale et particuliere, des mollusques, animaux sans vertèbres et a sang blanc. In: Dufart F Buffon L, et Sonnini CS (ed). Histoire général et particulière. Paris: Imprimerie, pp 38–52
Muss A, Robertson DR, Stepien CA, Wirtz P, Bowen BW (2001) Phylogeography of Ophioblennius: the role of ocean currents and geography in reef fish evolution. Evolution 55:561–572. https://doi.org/10.1554/0014-3820(2001)055[0561:POOTRO]2.0.CO;2
Naef A (1923) Cephalopoda. Part III. Fauna and Flora of the Gulf of Naples, Monograph no 35, part 2. Naples, pp 313–917
Nesis KN (1978) Comparison of cephalopod faunas along the coasts of Central America. Malacol Rev 11(1/2):127–128. https://doi.org/10.1080/04597237808460458
Norman MD, Hochberg FG, Finn JK (2014) Family Octopodidae. In Jereb P, Roper CFE, Norman MD, Finn JK (ed) Cephalopods of the World. An annotated and illustrated catalogue of cephalopod species known to date. Vol 3. Octopods and Vampire squids. Rome: Food and Agriculture Organization of the United Nations, pp 33–58
Okusu A, Schwabe E, Eernisse DJ, Giribet G (2003) Towards a phylogeny of chitons (Mollusca, Polyplacophora) based on combined analysis of five molecular loci. Org Divers Evol 3:281–302. https://doi.org/10.1078/1439-6092-00085
Opresko L, Thomas R (1975) Observations on Octopus joubini: some aspects of reproductive biology and growth. Mar Biol 31:51–61. https://doi.org/10.1007/BF00390647
Ortiz N, Ré ME, Márquez F (2006) First description of eggs, hatchlings and hatchling behaviour of Enteroctopus megalocyathus (Cephalopoda: Octopodidae). J Plankt Res 28:881–890. https://doi.org/10.1093/plankt/fbl023
Palacio FJ (1977) A study of coastal Cephalopods from Brazil with a review of Brazilian zoogeography. Dissertation, University of Miami
Perez JAA, Haimovici M (1991) Cephalopod collection of “Museu de Zoologia of Universidade de São Paulo”, São Paulo, Brazil. Papéis Avulsos De Zoologia 37(16):251–258
Perrier E, Rochebrune AT (1894) Sur octopus nouveau de la basse Californie, habitant les coquilles des Mollusques bivalves. Comptes Rendus des Seances de L’Academie Des Sciences 118:770–773
Pickford GE (1945) Le poulpe Américaine: a study of the littoral octopoda of the western Atlantic. Trans Connecticut Acad Arts Sci 36:701–811
Pickford GE (1946) A review of the littoral Octopoda from the central and western Atlantic stations on the collections of the British Museum. Ann Mag Nat Hist 13:412–429. https://doi.org/10.1080/00222934608654564
Pickford GE, McConnaughey BH (1949) The Octopus bimaculatus problem: a study in sibling species. B Bingham Oceanogr 12(4):1–66
Pliego-Cárdenas R, Hochberg FG, De LFJG, Barriga-Sosa IDLA (2014) Close genetic relationships between two American octopuses: Octopus hubbsorum Berry, 1953, and Octopus mimus Gould, 1852. J Shellfish Res 33:293–303
Posada D (2008) jModelTest: phylogenetic model averaging. Molec Biol Evol 25:1253–1256. https://doi.org/10.1093/molbev/msn083
Rambaut A, Suchard MA, Xie D, Drummond AJ (2014) Tracer v1.6. https://tree.bio.ed.ac.uk/software/tracer/. Accessed on 13 Jun 2019
Ritschard EA, Guerrero–Kommritz J, Sanchez JA (2019) First molecular approach to the octopus fauna from the southern Caribbean. PeerJ 7:e7300. https://doi.org/10.7717/peerj.7300
Robson GC (1929) A monograph of the recent Cephalopoda. Part I. Octopodinae. London: British Museum
Rocha LA (2003) Patterns of distribution and processes of speciation in Brazilian reef fishes. J Biogeog 30:1161–1171. https://doi.org/10.1046/j.1365-2699.2003.00900.x
Roper CFE, Voss GL (1983) Guidelines for taxonomic description of cephalopod species. In: Roper CFE, Lu CC, Hochberg FG (ed) Memoirs of the National Museum of Victoria: Proceedings of the workshop on the biology and resource potential of cephalopods, Melbourne, pp 48–64. https://doi.org/10.24199/j.mmv.1983.44.03
Shen Y, Kang J, Chen W, He S (2016) DNA barcoding for the identification of common economic aquatic products in Central China and its application for the supervision of the market trade. Food Control 61:79–91. https://doi.org/10.1016/j.foodcont.2015.08.038
Strugnell JM, Norman MD, Vecchione M, Guzik M, Allcock AL (2013) The ink sac clouds octopod evolutionary history. Hydrobiologia 725:215–235. https://doi.org/10.1007/s10750-013-1517-6
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Tiffany BN, Fangue NA, Bennett WA (2006) Disappearance of a population of pygmy octopus following a harmful algal bloom in a northwestern Florida bay, USA. Am Malacol Bull 21:11–15
Vaske–Jr T, Costa FAP (2011) Lulas e Polvos da Costa Brasileira. Fortaleza: Labomar UFC
Verany JB (1851) Mollusques méditerranéens, observés, decrits, figureś et chromolithographieś d'après nature: 1ère partie. Cephalopodes de la Méditerraneé. Imprimerie des Sourds-Muets. https://doi.org/10.5962/bhl.title.49684
Vidal EAG, Zeidberg LD, Buskey EJ (2018) Development of swimming abilities in squid paralarvae: behavioral and ecological implications for dispersal. Front Physiol 9:954. https://doi.org/10.3389/fphys.2018.00954
Villanueva R, Norman M (2018) Biology of the planktonic stages of benthic octopuses. Ocean Mar Biol Ann Rev 46:105–202. https://doi.org/10.1201/9781420065756.ch4
Villanueva R, Vidal EAG, Fernandez-Alvarez FA, Nabhitabhata J (2016) Early mode of life and hatchling size in cephalopod molluscs: influence on the species distributional ranges. PLoS ONE 11:1–27. https://doi.org/10.1371/journal.pone.0165334
Voight JR (1988) Trans-Panamanian geminate octopods (Mollusca: Octopoda). Malacol 29(1):289–294
Voight JR (1990) Population biology of Octopus digueti and the morphology of American tropical octopods. Dissertation, University of Arizona
Voight JR (1992) Movement, injuries and growth of members of a natural population of the Pacific pygmy octopus, Octopus digueti. J Zool 228:247–326. https://doi.org/10.1111/j.1469-7998.1992.tb04606.x
Voight JR (1998) An overview of shallow water octopus biogeography. In: Voss NA, Vecchione M, Toll RB (eds) Systematic and Biogeography of Cephalopods, vol II. Smithsonian Contributions to Zoology, Washington, pp 549–559
Voss GL (1950) Two new species of Cephalopods from the Florida Keys. Rev de la Socied Malacol “Carlos de la Torre” 7(2):73–79
Voss GL (1951) Further description of Octopus burryi Voss with a note on its distribution. Bull Mar Sci Gulf Carib 1(3):231–240
Voss GL (1968) Octopods from the R/V Pillsbury southern Caribbean Cruise, 1966, with a description of a new species, Octopus zonatus. Bull Mar Sci 18(3):645–659
Voss GL, Toll RB (1998) The systematics and nomenclatural status of the Octopodidae described from the western Atlantic Ocean. In: Voss NA, Vecchione M, Toll RB, Sweeney MJ (eds) Systematic and Biogeography of Cephalopods, vol II. Washington: Smithsonian Contributions to Zoology, pp 457–474
Zulueta CC (2019) Hermit crabs as emergent icons of global waste epidemic and their unreal estate housing struggles. Soc Anim 27:697–715. https://doi.org/10.1163/15685306-00001839
Acknowledgements
This paper is dedicated to Dr Eric Hochberg who encouraged one of us (TL) to study the pygmy octopus fauna of Brazil deeper, and for his valuable contribution and comments during my first visit at BMNH, and also Vanessa Delnavaz, who welcome us during our second visit to the SBMNH; Dr Michael Vecchione and Dr Jon Ablett for their welcome for TL at the Smithsonian Museum and Natural History Museum London respectively. We are thankful to Prof. Dr. Cristiano Albuquerque and Maria da Conceição Leite Spencer for help in measurement of specimens and counting endless octopus’ suckers during the museum’s visits, to Letícia Cavole for drawing our holotype and others biological structures, to the reviewers for their important suggestions and comments, to Abudefduf Atividades Subaquáticas and Juliana Valverde for field support in Ilha Grande, and to Ed Bastos for photos and information about the specimens found in the BG500 project.
Funding
This study was funded by the Brazilian National Council for Scientific and Technological Development and Coordination for the Improvement of Higher Education Personnel (Grants CNPq 481492/2013–9 and CAPES/Ciências do Mar II 2203/2014–01; 23038004807/2014–1). For financial support EAGV, SMQL, MH are research fellows from Brazilian National Council for Scientific and Technological Development (Grants 312331/2018–1, and 313644/2018–7, 307994/2020–respectively).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
International and national guidelines for the care and use of cephalopods were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the appropriate authorities (Instituto Chico Mendes de Conservação da Biodiversidade, ICMBio: License number 304841).
Data availability
The sequences generated and analyzed during the current study were submitted to GenBank repository. GenBank accession numbers are provided in the text.
Authors’ contributions
TL and MH conceived the ideas; TL, MH, SM and FL designed the methodology and analyzed the data; TL SM, FL, RD and GG collected the data and described the habitat and living behaviors; TL, MH, DV described the species morphologically and worked on figures and drawings; FL and SL, provided molecular data and analyses; EV analyzed eggs and hatchlings data, described the early life stages and prepared their drawings; JM participated in forming ideas, writing and reviewed all manuscript components. All authors contributed to writing of the drafts and final manuscript submitted for publication.
Additional information
Communicated by M. Vecchione
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is registered in ZooBank under http://zoobank.org/C6464E5F-F16F-4A62-9BF0-15BE8ACCAA82
Rights and permissions
About this article
Cite this article
Leite, T.S., Vidal, E.A.G., Lima, F.D. et al. A new species of pygmy Paroctopus Naef, 1923 (Cephalopoda: Octopodidae): the smallest southwestern Atlantic octopod, found in sea debris. Mar. Biodivers. 51, 68 (2021). https://doi.org/10.1007/s12526-021-01201-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-021-01201-z