Abstract
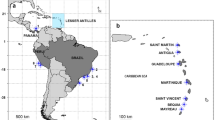
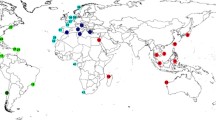
The Indo-Pacific is the world’s largest marine biogeographic region, covering the tropical and subtropical waters from the Red Sea in the Western Indian Ocean to the Easter Islands in the Pacific. It is characterized by a vast degree of biogeographic connectivity in particular in its marine realm. So far, usage of molecular tools rejected the presence of cosmopolitan or very widespread sponge species in several cases, supporting hypotheses on a higher level of endemism among marine invertebrates than previously thought. We analysed the genetic diversity of Hyrtios erectus and Stylissa massa, two alleged widespread sponge species of the Indo-Pacific, from the Red Sea and Mayotte in the West Indian Ocean to Polynesia in the Central Pacific. In the region of its type locality, the Red Sea, Hyrtios erectus is genetically distinct, and the populations from the remaining Indo-Pacific are a potentially different species and paraphyletic in respect to H. altus. Stylissa massa falls into different, but widespread genetic clades, one of them (Stylissa cf. massa), with distinct potentially hairpin-forming elements in mitochondrial intergenic regions. The results also indicate that morphologically established demosponge species in the Indo-Pacific can be widespread, but simultaneously harbour cryptic, genetically distinct lineages.



Similar content being viewed by others
References
Abdul Wahab MA, Fromont J, Whalan S et al (2014) Combining morphometrics with molecular taxonomy: how different are similar foliose keratose sponges from the Australian tropics? Mol Phylogenet Evol 73:23–39
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with image. J Biophotonics Int 11:36–42
Bailey G (2010) The Red Sea, coastal landscapes, and hominin dispersals. In: Petraglia MD, Rose JI (eds) The Evolution of Human Populations in Arabia. Springer, Netherlands, pp 15–37
Barnes DKA, Bell JJ (2002) Coastal sponge communities of the West Indian Ocean: taxonomic affinities, richness and diversity. Afr J Ecol 40:337–349
Bell JJ (2008) The functional roles of marine sponges. Estuar Coast Shelf Sci 79:341–353
Bierne N, Bonhomme F, David N (2003) Habitat preference and the marine-speciation paradox. Proc R Soc Lon, Biol Sci 270:1399–1406
Briggs JC (1987) Biogeography and Plate Tectonics. Elsevier Science
Carballo JL, Aguilar-Camacho JM, Knapp IS, Bell JJ (2013) Wide distributional range of marine sponges along the Pacific Ocean. Mar Biol Res 9:768–775
Carter HJ (1887) Report on the marine sponges, chiefly from King Island in the Mergui archipelago, collected for the trustees of the Indian museum, Calcutta, by Dr. John Anderson, FRS, superintendent of the museum. J Linnean Soc Lon, Zool 21:61–84
Cleary DFR, de Voogd NJ, Polónia ARM et al (2015) Composition and predictive functional analysis of bacterial communities in seawater, sediment and sponges in the Spermonde archipelago, Indonesia. Microb Ecol 70:889–903
Cox CB, Moore PD (2000) Biogeography: An Ecological and Evolutionary Approach. Blackwell Science
de Monte Lamarck JBP (1815) Suite des polypiers empâtés. Mémoirs du Muséum d’Histoire naturelle. Paris 1:69–80 162–168, 331–340
DiBattista J, Berumen ML, Gaither MR et al (2013) After continents divide: comparative phylogeography of reef fishes from the Red Sea and Indian Ocean. J Biogeogr 40:1170–1181
DiBattista J, Howard Choat J, Gaither MR et al (2016) On the origin of endemic species in the Red Sea. J Biogeogr 43:13–30
Erpenbeck D, Voigt O, Gültas M, Wörheide G (2008) The sponge genetree server-providing a phylogenetic backbone for poriferan evolutionary studies. Zootaxa 1939:58–60
Erpenbeck D, Voigt O, Wörheide G, Lavrov DV (2009) The mitochondrial genomes of sponges provide evidence for multiple invasions by repetitive hairpin-forming elements (RHE). BMC Genomics 10:591
Erpenbeck D, Hooper J, Bonnard I et al (2012a) Evolution, radiation and chemotaxonomy of Lamellodysidea, a demosponge genus with anti-plasmodial metabolites. Mar Biol 159:1119–1127
Erpenbeck D, Sutcliffe P, de SC C et al (2012b) Horny sponges and their affairs: on the phylogenetic relationships of keratose sponges. Mol Phylogenet Evol 63:809–816
Erpenbeck D, Ekins M, Enghuber N et al (2016a) Nothing in (sponge) biology makes sense – except when based on holotypes. J Mar Biol Ass 96:305–311
Erpenbeck D, Voigt O, Al-Aidaroos AM et al (2016b) Molecular biodiversity of Red Sea demosponges. Mar Pollut Bull 105:507–514
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and windows. Mol Ecol Resour 10:564–567
Francis WR, Eitel M, Vargas S et al (2016) Mitochondrial genomes of the freshwater sponges Spongilla lacustris and Ephydatia Cf. muelleri. Mitochondrial DNA Part B 1:250–251
Gordon AL (2005) Oceanography of the Indonesian seas and their throughflow. Oceanography 18:15–27
Gordon AL, Fine RA (1996) Pathways of water between the Pacific and Indian oceans in the Indonesian seas. Nature 379:146–149
Hooper JNA (2000) Sponguide: guide to sponge collection and identification. Queensland Museum. 26 pp. Downloadable from: www.southbank.qm.qld.gov.au/Find+out+about/Animals+of+Queensland/Sea+Life/Sponges/~/media/EC746F1940824D6BB350092FD8CAF53A.ashx
Hooper JNA, Lévi C (1994) Biogeography of indo-west Pacific sponges: Microcionidae, Raspailidae, Axinellidae. In: Van Soest RWM, Van Kempen TMG, Braekman JC (eds) Sponges in time and space. Balkema, Rotterdam, pp 191–212
Hooper JNA, Hall KA, Ekins M et al (2013) Managing and sharing the escalating number of sponge “unknowns”: the SpongeMaps project. Integr Comp Biol 53:473–481
Imešek M, Pleše B, Lukić-Bilela L et al (2012) Mitochondrial genomes of the genus Ephydatia Lamouroux, 1816: can palindromic elements be used in species-level studies? Org Divers Evol 13:127–134
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Kearse M, Moir R, Wilson A et al (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Keller C (1889) Die Spongienfauna des Rothen Meeres. I Hälfte Zeitschrift für Wissenschaftliche Zoologie 48:311–406
Kelly Borges M, Robinson EV, Gunasekera SP et al (1994) Species differentiation in the marine sponge genus Discodermia (Demospongiae: Lithistida): the utility of ethanol extract profiles as species specific chemotaxonomic markers. Biochem Syst Ecol 22:353–365
Kemp J (1998) Zoogeography of the coral reef fishes of the Socotra archipelago. J Biogeogr 25:919–933
Klausewitz W (1989) Evolutionary history and zoogeography of the Red Sea ichthyofauna. Fauna of Saudi Arabia 10:310–337
Klautau M, Russo CAM, Lazoski C et al (1999) Does cosmopolitanism result from overconservative systematics? A case study using the marine sponge Chondrilla nucula. Evolution 53:1414–1422
Lavrov DV (2010) Rapid proliferation of repetitive palindromic elements in mtDNA of the endemic Baikalian sponge Lubomirskia baicalensis. Mol Biol Evol 27:757–760
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Maldonado M (2006) The ecology of the sponge larva. Can J Zool 84:175–194
Maldonado M, Uriz MJ (1999) Sexual propagation by sponge fragments. Nature 398:476–476
Maldonado M, Carmona MC, Uriz MJ, Cruzado A (1999) Decline in Mesozoic reef-building sponges explained by silicon limitation. Nature 401:785–788
Markmann M, Tautz D (2005) Reverse taxonomy: an approach towards determining the diversity of meiobenthic organisms based on ribosomal RNA signature sequences. Philos Trans R Soc Lond Ser B Biol Sci 360:1917–1924
Palumbi SR, Grabowsky G, Duda TF Jr et al (1997) Speciation and population genetic structure in tropical Pacific sea urchins. Evolution 51:1506–1517
Paquin B, Laforest MJ, Lang BF (2000) Double-hairpin elements in the mitochondrial DNA of allomyces: evidence for mobility. Mol Biol Evol 17:1760–1768
Polónia ARM, Cleary DFR, Freitas R et al (2015) The putative functional ecology and distribution of archaeal communities in sponges, sediment and seawater in a coral reef environment. Mol Ecol 24:409–423
Pöppe J, Sutcliffe P, Hooper JNA et al (2010) COI barcoding reveals new clades and radiation patterns of indo-Pacific sponges of the family Irciniidae (Demospongiae: Dictyoceratida). PLoS One 5:e9950
Redmond NE, Morrow CC, Thacker RW et al (2013) Phylogeny and systematics of Demospongiae in light of new small-subunit ribosomal DNA (18S) sequences. Integr Comp Biol 53:388–415
Reveillaud J, van Soest RWM, Derycke S et al (2011) Phylogenetic relationships among NE Atlantic Plocamionida Topsent (1927) (Porifera, Poecilosclerida): under-estimated diversity in reef ecosystems. PLoS One 6:e16533
Rodriguez-Lanetty M, Hoegh-Guldberg O (2002) The phylogeograohy and connectivity of the latitudinally widespread scleractinian coral Pleisiastrea versipora in the western Pacific. Mol Ecol 11:1177–1189
Rosenthal A, Coutelle O, Craxton M (1993) Large-scale production of DNA sequencing templates by microtitre format PCR. Nucleic Acids Res 21:173–174
Rua CPJ, Zilberberg C, Sole-Cava AM (2011) New polymorphic mitochondrial markers for sponge phylogeography. J Mar Biol Assoc UK 91:1015–1022
Setiawan E, De Voogd NJ, Swierts T et al (2015) MtDNA diversity of the Indonesian giant barrel sponge Xestospongia testudinaria (Porifera: Haplosclerida) - implications from partial cytochrome oxidase 1 sequences. J Mar Biol Ass 96:323–332
Setiawan E, de Voogd NJ, Wörheide G, Erpenbeck D (2016) Bottomless barrel-sponge species in the indo-Pacific? Zootaxa 4136:393–396
Shorthouse DP (2010) SimpleMappr, an online tool to produce publication-quality point maps. Retrieved from http://www.simplemappr.net
Siddall M, Rohling EJ, Almogi-Labin A et al (2003) Sea-level fluctuations during the last glacial cycle. Nature 423:853–858
Solé-Cava AM, Boury-Esnault N (1999) Patterns of intra and interspecific genetic divergence in marine sponges. In: Hooper JNA (ed) Memoirs of the Queensland museum, Proceedings of the 5th international sponge symposium, Brisbane, pp 591–602
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313
Swierts T, Peijnenburg KTCA, de Leeuw C et al (2013) Lock, stock and two different barrels: comparing the genetic composition of morphotypes of the indo-pacific sponge Xestospongia testudinaria. PLoS One 8:e74396
Syamsudin F, van Aken HM, Kaneko A (2010) Annual variation of the southern boundery current in the Banda Sea. Dynamic Atmosph Oceans 50:129–139
Van Soest RWM (1994) Demosponge distribution patterns. In: Van Soest RWM, van Kempen TMG, Braekman J-C (eds) Sponges in time and space. Balkema, Rotterdam, pp 213–224
Van Soest RWM, Boury-Esnault N, Vacelet J et al (2012) Global diversity of sponges (Porifera). PLoS One 7:e35105
Voigt O, Wörheide G (2016) A short LSU rRNA fragment as a standard marker for integrative taxonomy in calcareous sponges (Porifera: Calcarea). Org Divers Evol 16:53–64
Voigt O, Erpenbeck D, González-Pech RA, et al (2017) Calcinea of the Red Sea: providing a DNA barcode inventory with description of four new species. Mar Biodivers 1–26
Williams ST, Benzie JAH (1996) Genetic uniformity of widely separated populations on the coral reef starfish Linckia laevigata from the east Indian and West Pacific oceans, revealed by allozyme electrophoresis. Mar Biol 126:99–107
Wörheide G (1998) The reef cave dwelling ultraconservative coralline demosponge Astrosclera willeyana Lister 1900 from the indo-Pacific - micromorphology, ultrastructure, biocalcification, isotope record, taxonomy, biogeography, phylogeny. Facies 38:1–88
Wörheide G, Degnan BM, Hooper JNA, Reitner J (2002) Biogeography and taxonomy of the Indo-Pacific reef cave dwelling coralline demosponge Astrosclera 'willeyana': new data from nuclear internal transcribed spacer sequences. In: Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia 23–27 October 2000 (eds. Moosa KM, Soemodihardjo S, Soegiarto A, et al.), pp. 339–345. State Ministry for the Environment, Indonesia, Indonesian Institute of Sciences & The International Society for Reef Studies, Bali, Indonesia
Wörheide G, Epp L, Macis L (2008) Deep genetic divergences among indo-Pacific populations of the coral reef sponge Leucetta chagosensis (Leucettidae): founder effects, vicariance, or both? BMC Evol Biol 8:24
Xavier JR, Rachello-Dolmen PG, Parra-Velandia FJ et al (2010) Molecular evidence of cryptic speciation in the “cosmopolitan” excavating sponge Cliona celata (Porifera, Clionaidae). Mol Phylogenet Evol 56:13–20
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415
Acknowledgements
DE and GW thank the Deutsche Forschungsgemeinschaft (DFG) for financial support (Er611/3-1, Wo896/15-1). RA thanks the Indonesian Government for funding of her work. The scientific research cooperation between King Abdulaziz University (KAU), Faculty of Marine Sciences (FMS), Jeddah, Saudi Arabia, and the Senckenberg Research Institute (SRI), Frankfurt, Germany, in the framework of the Red Sea Biodiversity Project, during which the present material was collected, was funded by KAU GRANT NO. “I/1/432-DSR”. The authors acknowledge, with many thanks, KAU and SRI for technical and financial support. Steve C. de Cook, Gabriele Büttner, Simone Schätzle and two anonymous reviewers are thanked for various contributions to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. M. El-Sherbiny
Electronic supplementary material
Supplementary Figure 1
Maximum likelihood phylogram of Hyrtios spp. based on the 28S data set. Note that positions with intragenomic polymorphisms were omitted prior to reconstructing the tree. Numbers on branches represent bootstrap support >70. Numbers preceding the taxon names refer to NCBI Genbank accession numbers or museum voucher numbers: (QM)G3#####: Queensland Museum; (SNSB-BSPG.)GW#### and others: Bavarian State Collection of Palaeontology and Geology (PDF 198 kb)
Supplementary Figure 2
Measurements of spicule length and width of selected representatives of S. massa and S. cf. massa. (PDF 103 kb)
Rights and permissions
About this article
Cite this article
Erpenbeck, D., Aryasari, R., Benning, S. et al. Diversity of two widespread Indo-Pacific demosponge species revisited. Mar Biodiv 47, 1035–1043 (2017). https://doi.org/10.1007/s12526-017-0783-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-017-0783-3