Abstract
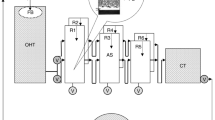
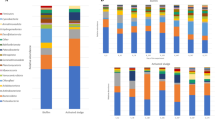
A biological aerated filter (100 l), filled with bamboo ball media, was set up for treatment of low ammonia-containing recirculating water in a marine aquaculture system. Chemical analysis showed that it took 70 days to establish a stable efficiency, at which more than 30% of the ammonia was removed. During the biofilm development, bacterial diversity and community structure were determined by construction of 16S rRNA gene libraries. Bacterial diversity and population richness were quite abundant and increased gradually, as revealed by the indexes of Shannon (from 3.34 to 3.94), Simpson (from 0.94 to 0.97) and Margalef (from 7.91 to 14.25). Dominant groups in the whole process showed a regular variation trend: a decrease of uncultured bacteria and Bacteroidetes from 38% to 32% and from 18% to 7%, respectively; and an increase of Alphaproteobacteria from 22% to 41%. In particular, clones closely relating to Denitromonas increased from 1.7% to 6.5% and then to 10% in the libraries A, B and C, respectively; in addition to the only detection of six clones (3%) representing Nitrospira in library C. Furthermore, a few strains capable of heterotrophic ammonia-oxidizing or aerobic denitrifying were isolated from the late phase of the biofilm development. All these results might infer that the biofilm bacterial community gradually shifts from chemical oxygen demand (COD)-removing flora to COD- and nitrogen-removing ones with the biofilm development, and that nitrification, heterotrophic ammonia oxidizing and denitrification all contribute to nitrogen removal of the biofilter.







Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
APHA, AWWA, WPCF (1998) Standards methods for the examination of water and wastewater, 9th edn. American Public Health Association, American Water Works Association, Water Pollution Control Facility, Washington
Avnimelech Y, Mozes N, Diab S, Kochba M (1995) Rates of organic carbon and nitrogen degradation in intensive fish ponds. Aquaculture 134:211–216
Avnimelech Y (1999) Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 176:227–235
Biesterfeld S, Russell P, Figueroa L (2003) Linking nitrifying biofilm structure and function through flouresent in situ hybridization and evolution of nitrification capacity. Water Environ Res 75:205–15
Boon N, Windt WD, Verstraete W (2002) Evaluation of nested PCR-DGGE (denaturing gradient gel electrophoresis) with group-specific 16S rRNA primers for the analysis of bacterial communities from different wastewater treatment plants. FEMS Microbiol Ecol 39:101–112
Bruhn JB, Nielsen KF, Hjelm M, Hansen M, Bresciani J, Schulz S, Gram L (2002) Ecology, inhibitory activity, and morphogenesis of a marine antagonistic bacterium belonging to the Roseobacter clade. Appl Environ Microbiol 71:7263–7270
Charest MH, Antoun H, Beauchamp CJ (2004) Dynamics of water-soluble carbon substances and microbial populations during the composting of de-inking paper sludge. Bioresour Technol 91:53–67
Chen CL, Liu WT, Chong ML, Wong MT, Ong SL, Seah H, Ng WJ (2004) Community structure of microbial biofilms associated with membrane-based water purification processes as revealed using a polyphasic approach. Appl Microbiol Biotechnol 63:466–473
Colt JE, Armstrong DA (1981) Nitrogen toxicity to crustaceans, fish, and mollusks. In: Proceedings of the Bioengineering Symposium for Fish Culture. Fish Culture Section, American Fisheries Society, Bethesda, pp 34–47
Dang H, Lovell CR (2000) Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified rRNA gene restriction analysis and sequence analysis of 16S rRNA genes. Appl Environ Microbiol 66:467–475
Fu SZ, Fan HX, Liu SJ, Liu Y, Liu ZP (2009) A bioaugmentation failure in biological filters caused by phage infection and weak biofilm formation ability. J Environ 21:1153–1161
Ioannis SA, Aikaterini K (2008) Fish industry waste: treatments, environmental impacts, current and potential uses. Int J Food Sci Technol 43:726–745
Itoi S, Ebihara N, Washio S, Sugita H (2007) Nitrite-oxidizing bacteria, Nitrospira, distribution in the outer layer of the biofilm from filter materials of a recirculating water system for the goldfish Carassius auratus. Aquaculture 264:297–308
Jensen HL (1952) The coryneform bacteria. Annu Rev Microbiol 6:77–90
Kim S, Kong I, Lee B, Kang L, Lee M, Suh K (2000) Removal of ammonium-N from a recirculation aquacultural system using an immobilized nitrifiers. Aquac Eng 21:139–150
Kumar S, Tamura K, Nei M (2004) MEGA 3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. John Wiley, Chichester, pp 115–175
Levin MA (1992) Microbial ecology: principle, methods and applications. McGraw-Hill, New York
Liu Y, Tay JH (2002) The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Res 36:1653–1665
Lyautey E, Ten-Hage L, Lacoste B, Rols JL, Garabetian F (2005) Analysis of bacterial diversity in river biofilms using 16S rDNA PCR-DGGE: methodological settings and fingerprints interpretation. Water Res 39:380–388
Margalef R (1958) Temporal succession and spatial heterogeneity in phytoplankton. In: Traverso B (ed) Perspectives in marine biology. University of California Press, Berkeley, pp 323–347
Martin-Laurent F, Philippot L, Hallet S, Chaussod R, Germon JC, Soulas G, Catroux G (2001) DNA Extraction from soils: old bias for new microbial diversity analysis methods. Appl Environ Microbiol 67:2354–2359
McNamara CJ, Leff LG (2004) Response of biofilm bacteria to dissolved organic matter from decomposing maple leaves. Microb Ecol 48:324–330
Meade JW (1985) Allowable ammonia for fish culture. Prog Fish Cult 47:135–145
Michaud L, Lo Giudice A, Troussellier M, Smedile F, Brunil V, Blancheton JP (2009) Phylogenetic characterization of the heterotrophic bacterial communities inhabiting a marine recirculating aquaculture system. J Appl Microbiol 107:1935–1936
Noophan P, Figueroa LA, Munakata-Marr J (2004) Nitrite oxidation inhibition by hydroxylamine: experimental and model evaluation. Water Sci Technol 50:295–304
Robertson LA, Cornelisse R, De Vos P, Hadioetomo R, Kuenen JG (1989) Aerobic denitrification in various heterotrophic nitrifiers. Antonie Leeuwenhoek 56:289–299
Rosa MF, Angela AL, Furtado RT (1998) Biofilm development and ammonia removal in the nitrification of a saline wastewater. Bioresour Technol 65:135–138
Saitou N, Nei M (1987) The neighbor-joining method: a new method for constructing phylogenetic trees. Mol Biol Evol 4:406–425
Sanz JL, Köchling T (2007) Molecular. biology techniques used in wastewater treatment: an overview. Process Biochem 42:119–133
Schramm A, Larsen LH, Revsbech NP, Ramsing NB, Amann R, Schleifer KH (1996) Structure and function of a nitrifying biofilm as determined by in situ hybridization and the use of microelectrodes. Appl Environ Microbiol 62:4641–4647
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–423
Simpson EH (1949) Measurement of diversity. Nature 163:688
Summerfelt ST (2006) Design and management of conventional fluidized-sand biofilters. Aquac Eng 34:275–302
Tal Y, Watts JEM, Schreier SB, Sowers KR, Schreier HJ (2003) Characterization of the microbial community and nitrogen transformation processes associated with moving bed bioreactors in a closed recirculated mariculture system. Aquaculture 215:187–202
Wimpenny J, Manz W, Szewzyk U (2000) Heterogeneity in biofilms. FEMS Microbiol Rev 24:661–671
Wortman B, Wheaton FW (1991) Temperature effects on biodrum nitrification. Aquacult Eng 10:183–205
Acknowledgements
This work was supported by grants from the high-tech development program of China (‘863’ program no. 2006AA100305).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, XY., Xu, Y., Liu, Y. et al. Bacterial diversity, community structure and function associated with biofilm development in a biological aerated filter in a recirculating marine aquaculture system. Mar Biodiv 42, 1–11 (2012). https://doi.org/10.1007/s12526-011-0086-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-011-0086-z