Abstract
Background
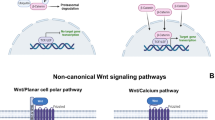
Bone remodeling is a lifelong process due to the balanced activity of osteoclasts (OCs), the bone-reabsorbing cells, and osteoblasts (OBs), and the bone-forming cells. This equilibrium is regulated by numerous cytokines, but it has been largely demonstrated that the RANK/RANKL/osteoprotegerin and Wnt/β-catenin pathways play a key role in the control of osteoclastogenesis and osteoblastogenesis, respectively. The pro-osteoblastogenic activity of the Wnt/β-catenin can be inhibited by sclerostin and Dickkopf-1 (DKK-1). RANKL, sclerostin and DKKs-1 are often up-regulated in bone diseases, and they are the target of new monoclonal antibodies.
Data sources
The authors performed a systematic literature search in PubMed and EMBASE to June 2018, reviewed and selected articles, based on pre-determined selection criteria.
Results
We re-evaluated the role of RANKL, osteoprotegerin, sclerostin and DKK-1 in altered bone remodeling associated with some inherited and acquired pediatric diseases, such as type 1 diabetes mellitus (T1DM), alkaptonuria (AKU), hemophilia A, osteogenesis imperfecta (OI), 21-hydroxylase deficiency (21OH-D) and Prader–Willi syndrome (PWS). To do so, we considered recent clinical studies done on pediatric patients in which the roles of RANKL-RANK/osteoprotegerin and WNT-ß-catenin signaling pathways have been investigated, and for which innovative therapies for the treatment of osteopenia/osteoporosis are being developed.
Conclusions
The case studies taken into account for this review demonstrated that quite frequently both bone reabsorbing and bone deposition are impaired in pediatric diseases. Furthermore, for some of them, bone damage began in childhood but only manifested with age. The use of denosumab could represent a valid alternative therapeutic approach to improve bone health in children, although further studies need to be carried out.


Similar content being viewed by others
References
Lu J, Shin Y, Yen MS, Sun SS. Peak bone mass and patterns of change in total bone mineral density and bone mineral contents from childhood into young adulthood. J Clin Densitom. 2016;19:180–91.
Massey HM, Flanagan AM. Human osteoclasts derive from CD14-positive monocytes. Br J Haematol. 1999;106:167–70.
Boyle WJ, Scott Simonet W, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42.
Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 2002;20:795–823.
Krishnan V, Bryant HU, Mac Dougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–9.
Reya T, Clevers H. Wnt signalling in stem cell and cancer. Nature. 2005;434:843–50.
Kato M, Patel MS, Lavasseur R, Lobov I, Chang BH, Glass DA, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:763–71.
Bodine PV, Zhao W, Kharode Y, Bex FJ, Lambert AJ, Goad MB, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–37.
Morvan F, Boulukos K, Clément-Lacroix P, Roman Roman S, Suc- Royer I, Vayssière B, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21:934–45.
Delgado-Calle J, Sato AJ, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37.
Baron R, Kneissel M. Wnt signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:1791–2.
Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J, et al. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008;112:196–207.
Dalbeth N, Pool B, Smith T, Callon KE, Lobo M, Taylor WJ, et al. Circulating mediators of bone remodeling in psoriatic arthritis: implications for disordered osteoclastogenesis and bone erosion. Arthritis Res Ther. 2010;12:R164.
Pan H, Wu N, Yang T, He W. Association between bone mineral density and type 1 diabetes mellitus: a meta analysis of cross sectional studies. Diabetes Metab Res Rev. 2014;30:531–42.
Bechtold S, Dirlenbach I, Raile K, Noelle V, Bonfing W, Schwarz HP. Early manifestation of type 1 diabetes in children is a risk factor for changed bone geometry: data using peripheral quantitative computed tomography. Pediatrics. 2006;118:627–34.
Weber DR, Haynes K, Leonard MB, Willi SM, Denburg MR. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population-based cohort study using The Health Improvement Network (THIN). Diabetes Care. 2015;38:1913–20.
Liao CC, Lin CS, Shih CC, Yeh CC, Chang YC, Lee YW, et al. Increased risk of fracture and post fracture adverse events in patients with diabetes: two nationwide population-based retrospective cohort studies. Diabetes Care. 2014;37:2246–52.
Fowlkes JL, Bunn RC, Thrailkill KM. Contributions of the insulin/insulin-like growth factor-1 axis to diabetic osteopathy. J Diabetes Metab. 2011;1:S1–003.
Fowlkes JL, Nyman JS, Bunn RC, Jo C, Wahl EC, Liu L, et al. Osteo-promoting effects of insulin-like growth factor I (IGF-1) in a mouse model of type 1 diabetes. Bone. 2013;57:36–40.
Botolin S, McCabe LR. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. 2006;99:411–24.
Neumann T, Lodes S, Kästner B, Franke S, Kiehntopf M, Lehmann T, et al. High serum pentosidine but not esRAGE is associated with prevalent fracture type 1 diabetes independent of bone mineral density and glycaemic control. Osteoporos Int. 2014;25:1527–33.
Tsentidis C, Gourgiotis D, Kossiva L, Doulgeraki A, Marmarinos A, Galli-Tsinopoulou A, et al. Higher levels of s-RANK-L and osteoprotegerin in children and adolescents with type 1 diabetes mellitus may indicate increased osteoclast signaling and predisposition to lower bone mass: a multivariate cross-sectional analysis. Osteoporos Int. 2016;27:1631–43.
Faienza MF, Ventura A, Delvecchio M, Fusillo A, Piacente L, Aceto G, et al. High sclerostin and Dickkopf-1 (DKK-1) serum levels in children and adolescents with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2017;102:1174–81.
Tsentidis C, Gourgiotis D, Kossiva L, Marmarinos A, Doulgeraki A, Karavanaki K. Increased levels of Dickkopf-1 (DKK-1) are indicative of Wnt/β catenin downregulation and lower osteoblast signaling in children and adolescents with type 1 diabetes mellitus, contributing to lower bone mineral density. Osteoporosis Intern. 2017;28:945–53.
Faienza MF, Brunetti G, Sanesi L, Colaianni G, Celi M, Piacente L, et al. High irisin levels are associated with better glycemic control and bone health in children with type 1 diabetes. Diabetes Res Clin Pract. 2018;141:10–7.
Natalicchio A, Marrano N, Biondi G, Spagnuolo R, Labarbuta R, Porreca I, et al. The myokine irisin is released in response to saturated fatty acids and promotes pancreatic β-cell survival and insulin secretion. Diabetes. 2017;66:2849–56.
Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA. 2015;112:12157–62.
Colaianni G, Mongelli T, Cuscito C, Pignataro P, Lippo L, Spiro G, et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci Rep. 2017;7:2811.
Tsentidis C, Gourgiotis D, Kossiva L, Marmarinos A, Doulgeraki A, Karavanaki K. Sclerostin distribution in children and adolescents with type 1 diabetes mellitus and correlation with bone metabolism and bone mineral density. Pediatr Diabetes. 2016;17:289–99.
Neumann T, Hofbauer LC, Rauner M, Lodes S, Kästner B, Franke S, et al. Clinical and endocrine correlates of circulating sclerostin levels in patients with type 1 diabetes mellitus. Clin Endocrinol. 2014;80:649–55.
Felício KM, de Souza ACCB, Neto JFA, de Melo FTC, Carvalho CT, Arbage TP, et al. Glycemic variability and insulin needs in patients with type 1 diabetes mellitus supplemented with vitamin D: a pilot study using continuous glucose monitoring system. Curr Diabetes Rev. 2018;14:395–403.
Al Hafid N, Christodoulou J. Phenylketonuria: a review of current and future treatments. Transl Pediatr. 2015;4:304–17.
Millucci L, Spreafico A, Tinti L, Braconi D, Ghezzi L, Paccagnini E, et al. Alkaptonuria is a novel human secondary amyloidogenic disease. Biochim Biophys Acta. 2012;1822:1682–16891.
Millucci L, Braconi D, Bernardini G, Lupetti P, Rovensky J, Ranganath L, et al. Amyloidosis in alkaptonuria. J Inherit Metab Dis. 2015;38:797–805.
Aliberti G, Pulignano I, Schiappoli A, Minisola S, Romagnoli E. Proietta M bone metabolism in ochronotic patients. J Intern Med. 2003;254:296–300.
Brunetti G, Tummolo A, D’amato G, Gaeta A, Ortolani F, Piacente L, et al. Mechanisms of enhanced osteoclastogenesis in alkaptonuria. Am J Pathol. 2018;188:1059–68.
Brunetti G, Rizzi R, Oranger A, Gigante I, Mori G, Taurino G, et al. LIGHT/TNFSF14 increases osteoclastogenesis and decreases osteoblastogenesis in multiple myeloma-bone disease. Oncotarget. 2014;5:12950–67.
Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–8.
Brunetti G, Faienza MF, Colaianni G, Gigante I, Oranger A, Pignataro P, et al. Impairment of bone remodeling in LIGHT/TNFSF14-deficient mice. J Bone Miner Res. 2018;33:704–19.
Christoforidis A, Economou M, Papadopoulou E, Kazantzidou E, Farmaki E, Tzimouli V, et al. Comparative study of dual energy X-ray absorptiometry and quantitative ultrasonography with the use of biochemical markers of bone turnover in boys with haemophilia. Haemophilia. 2011;17:217–22.
Katsarou O, Terpos E, Chatzismalis P, Provelengios S, Adraktas T, Hadjidakis D, et al. Increased bone resorption is implicated in the pathogenesis of bone loss in hemophiliacs: correlations with hemophilic arthropathy and HIV infection. Ann Haematol. 2010;89:67–74.
Giordano P, Brunetti G, Lassandro G, Notarangelo LD, Luciani M, Mura RM, et al. High serum sclerostin levels in children with haemophilia A. Br J Haematol. 2016;172:293–5.
Forlino A, Cabral WA, Barnes AM, Marini JC. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol. 2011;7:540–57.
Uveges TE, Collin-Osdoby P, Cabral WA, Ledgard F, Goldberg L, Bergwitz C, et al. Cellular mechanism of decreased bpne in Brtl mouse model of OI: imbalance of osteoblast function and increased osteoclasts and their precursors. J Bone Miner Res. 2008;23:1983–94.
Brunetti G, Papadia F, Tummolo A, Fischetto R, Nicastro F, Piacente L, et al. Impaired bone remodeling in children with osteogenesis imperfecta treated and untreated with bisphosphonates: the role of DKK1, RANKL, and TNF-α. Osteoporos Int. 2016;304:546–54.
Camacho NP, Raggio CL, Doty SB, Root L, Zraick V, Ilg WA, et al. A controlled study of the effects of alendronate in a growing mouse model of osteogenesis imperfecta. Calcif Tissue Int. 2001;69:94–101.
Evans KD, Lau ST, Oberbauer AM, Martin RB. Alendronate affects long bone length and growth plate morphology in the oim mouse model for osteogenesis imperfecta. Bone. 2003;2:268–74.
McCarthy EA, Raggio CL, Hossack MD, Miller EA, Jain S, Boskey AL, et al. Alendronate treatment for infants with osteogenesis imperfecta: demonstration of efficacy in a mouse model. Pediatr Res. 2002;52:660–70.
Delos D, Yang X, Ricciardi BF, Myers ER, Bostrom MP, Camacho NP. The effects of RANKL inhibition on fracture healing and bone strength in a mouse model of osteogenesis imperfecta. J Orthop Res. 2008;26:153–64.
Bargman R, Huang A, Boskey A, Raggio C, Pleshko N. RANKL inhibition improves bone properties in a mouse model of osteogenesis imperfecta. Connect Tissue Res. 2010;51:123–31.
Bargman R, Posham R, Boskey A, Carter E, DiCarlo E, Verdelis K, et al. High- and low-dose OPG-Fc cause osteopetrosis-like changes in infant mice. Pediatr Res. 2012;72:495–501.
Bargman R, Posham R, Boskey AL, DiCarlo E, Raggio C, Pleshko N. Comparable outcomes in fracture reduction and bone properties with RANKL inhibition and alendronate treatment in a mouse model of osteogenesis imperfecta. Osteoporos Int. 2012;23:1141–50.
Semler O, Netzer C, Hoyer-Kuhn H, Becker J, Eysel P, Schoenau E. First use of the RANKL antibody denosumab in osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact. 2012;12:183–8.
Joint LWPES/ESPE CAH Group. Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. J Clin Endocrinol Metab. 2002;87:4048–53.
Ventura A, Brunetti G, Colucci S, Oranger A, Ladisa F, Cavallo L, et al. Glucocorticoid-induced osteoporosis in children with 21-hydroxylase deficiency. Biomed Res Int. 2013;2013:250462.
Faienza MF, Brunetti G, Colucci S, Piacente L, Ciccarelli M, Giordani L, et al. Osteoclastogenesis in children with 21-hydroxylase deficiency on long-term glucocorticoid therapy: the role of receptor activator of nuclear factor κB ligand/osteoprotegerin imbalance. J Clin Endocrinol Metab. 2009;94:2269–76.
Abd El Dayem SM, Anwar GM, Salama H, Kamel AF, Emara N. Bone mineral density, bone turnover markers, lean mass, and fat mass in Egyptian children with congenital adrenal hyperplasia. Arch Med Sci. 2010;6:104–10.
Metwalley KA, El-Saied AR. Bone mineral status in Egyptian children with classical congenital adrenal hyperplasia. A single center study from Upper Egypt. Indian J Endocrinol Metab. 2014;18:700–4.
Brunetti G, Faienza MF, Piacente L, Ventura A, Oranger A, Carbone C, et al. High dickkopf-1 levels in sera and leukocytes from children with 21-hydroxylase deficiency on chronic glucocorticoid treatment. Am J Physiol Endocrinol Metab. 2013;304:546–54.
Butler MG, Manzardo AM, Forster JL. Prader–Willi syndrome: clinical genetics and diagnostic aspects with treatment approaches. Curr Pediatr Rev. 2016;12:136–66.
Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader–Willi syndrome. Genet Med. 2012;14:10–26.
Vestergaard P, Kristensen K, Bruun JM, Østergaard JR, Heickendorff L, Mosekilde L, et al. Reduced bone mineral density and increased bone turnover in Prader–Willi syndrome compared with controls matched for sex and body mass index—a cross-sectional study. J Pediatr. 2004;144:614–9.
Carrel AL, Myers SE, Whitman BY, Allen DB. Benefits of long-term GH therapy in Prader–Willi syndrome: a 4-year study. J Clin Endocrinol Metab. 2002;87:1581–15885.
Brunetti G, Grugni G, Piacente L, Delvecchio M, Ventura A, Giordano P, et al. Analysis of circulating mediators of bone remodelling in Prader–Willi syndrome. Calcific Tissue Int. 2018;102:635–43.
Faienza MF, Chiarito M, D’amato G, Colaianni G, Colucci S, Grano M, et al. Monoclonal antibodies for treating osteoporosis. Expert Opin Biol Ther. 2018;18:149–57.
Hoyer-Kuhn H, Franklin J, Allo G, Kron M, Netzer C, Eysel P, et al. Safety and efficacy of denosumab in children with osteogenesis imperfect-a first prospective trial. J Musculoskelet Neuronal Interact. 2016;16:24–32.
Funding
No funding
Author information
Authors and Affiliations
Contributions
GB and MF wrote the review; GD and MC revised the literature; AT, GC, SC and MG wrote the introduction and conclusion and critically revised the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants, but refers to previously published papers/studies that were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Rights and permissions
About this article
Cite this article
Brunetti, G., D’Amato, G., Chiarito, M. et al. An update on the role of RANKL–RANK/osteoprotegerin and WNT-ß-catenin signaling pathways in pediatric diseases. World J Pediatr 15, 4–11 (2019). https://doi.org/10.1007/s12519-018-0198-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-018-0198-7