Abstract
Background
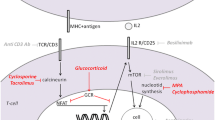
Mizoribine (MZR) is a selective inhibitor of inosine monophosphate dehydrogenase, a key enzyme in the pathway responsible for de novo synthesis of guanine nucleotides. As an immunosuppressant, MZR has been used successfully without any serious adverse effects in the treatment of renal diseases in children as well as adults. Besides its immunosuppressive effect, MZR has been reported to ameliorate tubulointerstitial fibrosis in rats via suppression of macrophage infiltration.
Data Sources
In this review, we summarize reported possible benefits of MZR in the treatment of pediatriconset glomerular disease.
Results
We recently observed that MZR itself selectively attenuates the expression of monocyte chemoattractant protein-1 at both the mRNA and protein levels in human mesangial cells. Since MZR binds specifically to 14-3-3 proteins and heat shock protein 60, both of which are reportedly expressed in inflamed glomeruli, MZR may bind directly to inflamed glomerular cells, thereby possibly preventing progressive damage from glomerulonephritis through a suppressive effect on activated macrophages and intrinsic renal cells. Moreover, it has recently been reported that MZR directly prevents podocyte injury through correction of the intracellular energy balance and nephrin biogenesis in cultured podocyte and rat models, suggesting a direct anti-proteinuric effect of MZR.
Conclusions
These beneficial mechanisms of action of MZR as well as its immunosuppressive effect would warrant its use in the treatment of pediatric-onset glomerular disease. Although further studies remain to be done, we believe that MZR may be an attractive treatment of choice for children with glomerular diseases from a histologic as well as clinical standpoint.
Similar content being viewed by others
References
Yokota S. Mizoribine: mode of action and effects in clinical use. Pediatr Int 2002;44:196–198.
Kawasaki Y. Mizoribine: a new approach in the treatment of renal disease. Clin Dev Immunol 2009;2009:681482.
Sonda K, Takahashi K, Tanabe K, Funchinoue S, Hayasaka Y, Kawaguchi H, et al. Clinical pharmacokinetic study of mizoribine in renal transplantation patients. Transplant Proc 1996;28:3643–3648.
Yoshioka K, Ohashi Y, Sakai T, Ito H, Yoshikawa N, Nakamura H, et al. A multicenter trial of mizoribine compared with placebo in children with frequently relapsing nephrotic syndrome. Kidney Int 2000;58:317–324.
Honda M. Nephrotic syndrome and mizoribine in children. Pediatr Int 2002;44:210–216.
Kawasaki Y, Hosoya M, Suzuki J, Onishi N, Takahashi A, Isome M, et al. Efficacy of multidrug therapy combined with mizoribine in children with diffuse IgA nephropathy in comparison with multidrug therapy without mizoribine and with methylprednisolone pulse therapy. Am J Nephrol 2004;24:576–581.
Yoshikawa N, Nakanishi K, Ishikura K, Hataya H, Iijima K, Honda M. Combination therapy with mizoribine for severe childhood IgA nephropathy: a pilot study. Pediatr Nephrol 2008;23:757–763.
Tanaka H, Tsugawa K, Tsuruga K, Suzuki K, Nakahata T, Ito E, et al. Mizoribine for the treatment of lupus nephritis in children and adolescents. Clin Nephrol 2004;62:412–417.
Yumura W, Suganuma S, Uchida K, Moriyama T, Otsubo S, Takei T, et al. Effects of long-term treatment with mizoribine in patients with proliferative lupus nephritis. Clin Nephrol 2005;64:28–34.
Shibasaki T, Matsuda H, Gomi H, Usui M, Ishimoto F, Sakai O. Mizoribine reduces urinary protein excretion in rats given puromycin aminonucleoside. Am J Nephrol 1996;16:167–172.
Takahashi S, Wakui H, Gustafsson JA, Zilliacus J, Itoh H. Functional interaction of the immunosuppressant mizoribine with the 14-3-3 protein. Biochem Biophys Res Commun 2000;274:87–92.
Tanaka H, Suzuki K, Nakahata T, Tsugawa K, Ito E, Waga S. Mizoribine oral pulse therapy for patients with disease flare of lupus nephritis. Clin Nephrol 2003;60:390–394.
Tanaka H, Tsugawa K, Suzuki K, Nakahata T, Ito E. Long-term mizoribine intermittent pulse therapy for young patients with flare of lupus nephritis. Pediatr Nephrol 2006;21:962–966.
Kuroda T, Hirose S, Tanabe N, Sato H, Nakatsue T, Ajiro J, et al. Mizoribine therapy for patients with lupus nephritis: the association between peak mizoribine concentration and clinical efficacy. Mod Rheumatol 2007;17:206–212.
Tanaka H, Oki E, Tsuruga K, Sato N, Matsukura H, Matsunaga A, et al. Mizoribine treatment of young patients with severe lupus nephritis: a clinicopathologic study by the tohoku pediatric study group. Nephron Clin Pract 2008;110:c73–c79.
Ohtomo Y, Fujinaga S, Takada M, Murakami H, Akashi S, Shimizu T, et al. High-dose mizoribine therapy for childhoodonset frequently relapsing steroid-dependent nephrotic syndrome with cyclosporin nephrotoxicity. Pediatr Nephrol 2005;20:1744–1749.
Kawasaki Y, Takano K, Isome M, Suzuki J, Suyama K, Kanno H, et al. Efficacy of single dose of oral mizoribine pulse therapy two times per week for frequently relapsing nephrotic syndrome. J Nephrol 2007;20:52–56.
Doi T, Masaki T, Shiraki N, Kawai T, Yorioka N. Oral mizoribine pulse therapy for steroid-dependent focal segmental glomerulosclerosis. Clin Nephrol 2008;69:433–435.
Fuke T, Abe Y, Hibino S, Takeshi M, Saito T, Sakurai S, et al. Mizoribine requires individual dosing due to variation of bioavailability. Pediatr Int 2012;54:885–891.
Ikezumi Y, Suzuki T, Karasawa T, Kawachi H, Nikolic-Paterson DJ, Uchiyama M. Use of mizoribine as a rescue drug for steroid-resistant pediatric IgA nephropathy. Pediatr Nephrol 2008;23:645–650.
Tanaka H, Oki ES, Tsugawa K, Suzuki K, Tsuruga K, Ito E. Long-term intermittent pulse therapy with mizoribine attenuates histologic progression in young patients with severe lupus nephritis: report of two patients. Nephrology (Carlton) 2007;12:376–379.
Tanaka H, Oki E, Tsuruga K, Aizawa-Yashiro T, Ito Y, Sato N, et al. Mizoribine attenuates renal injury and macrophage infiltration in patients with severe lupus nephritis. Clin Rheumatol 2010;29:1049–1054.
Sato N, Shiraiwa K, Kai K, Watanabe A, Ogawa S, Kobayashi Y, et al. Mizoribine ameliorates the tubulointerstitial fibrosis of obstructive nephropathy. Nephron 2001;89:177–185.
Kikuchi Y, Imakiire T, Yamada M, Saigusa T, Hyodo T, Hyodo N, et al. Mizoribine reduces renal injury and macrophage infiltration in non-insulin-dependent diabetic rats. Nephrol Dial Transplant 2005;20:1573–1581.
Takahashi S, Taniguchi Y, Nakashima A, Arakawa T, Kawai T, Doi S, et al. Mizoribine suppresses the progression of experimental peritoneal fibrosis in a rat model. Nephron Exp Nephrol 2009;112:e59–e69.
Itoh H, Komatsuda A, Wakui H, Miura AB, Tashima Y. Mammalian HSP60 is a major target for an immunosuppressant mizoribine. J Biol Chem 1999;274:35147–35151.
Tanaka H, Tsugawa K, Oki E, Suzuki K, Waga S, Ito E. Longterm mizoribine intermittent pulse therapy, but not azathioprine therapy, attenuated histologic progression in a patient with severe lupus nephritis. Clin Nephrol 2007;68:198–200.
Stypinski D, Obaidi M, Combs M, Weber M, Stewart AJ, Ishikawa H. Safety, tolerability and pharmacokinetics of higherdose mizoribine in healthy male volunteers. Br J Clin Pharmacol 2006;63:459–468.
Ikezumi Y, Suzuki T, Karasawa T, Hasegawa H, Kawachi H, Nikolic-Paterson DJ, et al. Contrasting effects of steroids and mizoribine on macrophage activation and glomerular lesions in rat thy-1 mesangial proliferative glomerulonephritis. Am J Nephrol 2010;31:273–282.
Anders HJ. Pseudoviral immunity-a novel concept for lupus. Trends Mol Med 2009;15:553–561.
Imaizumi T, Tanaka H, Matsumiya T, Yoshida H, Tanji K, Tsuruga K, et al. Retinoic acid-inducible gene-I is induced by double-stranded RNA and regulates the expression of CC chemokine ligand (CCL) 5 in human mesangial cells. Nephrol Dial Transplant 2010;25:3534–3539.
Imaizumi T, Aizawa-Yashiro T, Tsuruga K, Tanaka H, Matsumiya T, Yoshida H, et al. Melanoma differentiation-associated gene 5 regulates the expression of a chemokine CXCL10 in human mesangial cells: implications for chronic inflammatory renal diseases. Tohoku J Exp Med 2012;228:17–26.
Aizawa-Yashiro T, Imaizumi T, Tsuruga K, Watanabe S, Matsumiya T, Hayakari R, et al. Glomerular expression of fractalkine is induced by polyinosinic-polycytidylic acid in human mesangial cells: possible involvement of fractalkine after viral infection. Pediatr Res 2013;73:180–186.
Tanaka H, Imaizumi T. Inflammatory chemokine expression via Toll-like receptor 3 signaling in normal human mesangial cells. Clin Dev Immunol 2013;2013:984708.
Aizawa T, Imaizumi T, Tsuruga K, Watanabe S, Chiba Y, Matsumiya T, et al. Mizoribine selectively attenuates monocyte chemoattractant protein-1 production in cultured human glomerular mesangial cell: a possible benefit of its use in the treatment of lupus nephritis. Nephrology (Carlton) 2014;19:47–52.
Yamabe H, Shimada M, Murakami R, Fujita T, Shimaya Y, Nakamura N. Mizoribine suppresses proliferation of rat glomerular epithelial cells in culture and inhibits increase of monocyte chemoattractant protein-1 and macrophage inflammatory protein-2 stimulated by thrombin. Biol Pharm Bull 2012;35:705–708.
Uemura O, Ushijima K, Yamada T, Kimpara Y. Blood concentration and urinary excretion of mizoribine for internal use in pediatric kidney disease patients. Jpn J Pediatr Nephrol 2007;20:9–13. [In Japanese]
Takeuchi S, Hiromura K, Tomioka M, Takahashi S, Sakairi T, Maeshima A, et al. The immunosuppressive drug mizoribine directly prevents podocyte injury in puromycin aminonucleoside nephrosis. Nephron Exp Nephrol 2010;116:e3–e10.
Nakajo A, Khoshnoodi J, Takenaka H, Hagiwara E, Watanabe T, Kawakami H, et al. Mizoribine corrects defective nephrin biogenesis by restoring intracellular energy balance. J Am Soc Nephrol 2007;18:2554–2564.
Fujita T, Shimizu C, Ito K, Fuke Y, Satomura A, Matsumoto K. Anti-proteinuric effect of mizoribine monotherapy in three patients with membranous nephropathy. Clin Nephrol 2010;74:81–82.
Shimizu H, Takahashi M, Takeda S, Tahara K, Inoue S, Hakamata Y, et al. Effect of conversion from cyclosporine A to mizoribine on transplant arteriosclerosis in rat aortic allograft models. Microsurgery 2003;23:454–457.
Hara S, Umino D, Someya T, Fujinaga S, Ohtomo Y, Murakami H, et al. Protective effects of Mizoribine on Cyclosporine A nephropathy in rats. Pediatr Res 2009;66:524–527.
Aizawa-Yashiro T, Tsuruga K, Watanabe S, Oki E, Ito E, Tanaka H. Novel multidrug therapy for children with cyclosporineresistant or -intolerant nephrotic syndrome. Pediatr Nephrol 2011;26:1255–1261.
Hirose C, Aizawa T, Watanabe S, Tsuruga K, Ito E, Tanaka H. Efficacy of long-term multidrug therapy in a patient with focal segmental glomerulosclerosis. Pediatr Int 2014;56:129–130.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, H., Tsuruga, K. & Imaizumi, T. Mizoribine in the treatment of pediatric-onset glomerular disease. World J Pediatr 11, 108–112 (2015). https://doi.org/10.1007/s12519-015-0013-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-015-0013-7