Abstract
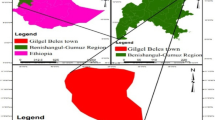
Shahre-Kord aquifer is located at the east part of Karoon basin. The water of Shahre-Kord aquifer has acceptable to unsuitable quality for drinking and also suitable and useful for the agricultural utilities. In this research, sampling from the studied aquifer was performed at spring and autumn. The obtained results of analysis performed on autumn samples revealed that the concentration of nitrate in the two wells was higher than its allowable limit in the drinking water. In the measurements of the samples at the end of spring, the highest concentration of nitrate from 16 samples belongs to the drinking water of Taghanak municipality which also has more than the allowable limit within the drinking water range, whereas the concentration of phosphate in all samples lies in the allowable limit. The samples were prepared through treatment by plant materials and different values of nanoscale zero-valent iron (NZVI) during 7 days. The measuring of nitrate concentration of samples illustrated that the south of aquifer have higher concentration in autumn than spring because N-fertilizers usually are used in autumn and the spring precipitation of nitrates. The results derived by several treatment methods in this study indicate that the combination of NZVI with fine pieces of corn for a period of 7 days is the best treatment, which has the highest efficiency for nitrate removal from an aquifer.







Similar content being viewed by others
References
Adebowale KO, Unuabonah IE, Olu-Owolabi BI (2006) The effect of some operating variables on the adsorption of lead and cadmium ions on kaolinite clay. J Hazard Mater 134:130–139
Alowitz MJ, Scherer MM (2002) Kinetics of nitrate, nitrite and Cr(VI) reduction by iron metal. Environ Sci Technol 36:299–306
Beltran de Heredia J, Domınguez JR, Cano Y, Jimenez I (2006) Nitrate removal from groundwater using Amberlite IRN-78: modelling the system. Appl Surf Sci 252:6031–6035
Bhatnagar A, Sillanpää M (2011) A review of emerging adsorbents for nitrate removal from water. Chem Eng J 168:493–504. https://doi.org/10.1016/j.cej.2011.01.103
Canter LW (1997) Nitrates in groundwater. CRC Press, Boca Raton
Cao S (2016) Studies on the reactivity activation of zero-valent iron (ZVI) with hydrogen peroxide for nitrate reduction in mine water. M.Sc thesis of degree Program of Chemical and Process Engineering, Lappeenranta University of Technology, School of Engineering Science
Chen YM, Li CW, Chen SS (2005) Fluidized zero valent iron bed reactor for nitrate removal. Chemosphere 59:753–759
Cheng IF, Muftikian R, Fernando Q, Korte N (1997) Reduction of nitrate to ammonia by zero valent iron. Chemosphere 35:2689–2695
Choe S, Chang Y, Hwang K, Khim J (2000) Kinetics of reductive denitrification by nanoscale zero-valent iron. Chemosphere 41:1307–1311
Choe S, Liljestrand HM, Kim J (2004) Nitrate reduction by zero-valent iron under different pH regimes. Appl Geochem 19:335–342
Crane RA, Scott TB (2012) Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. J Hazard Mater 211-212:112–125
Davis AP (2007) Field performance of bioretention: water quality. Environ Eng Sci 24:1048–1064
Di Palma L, Gueye MT, Petrucci E (2015) Hexavalent chromium reduction in contaminated soil: a comparison between ferrous sulphate and nanoscale zero-valent iron. J Hazard Mater 281:70–76
Dore M, Simon P, Deguin A, Victot J (1986) Removal of nitrate in drinking water by ion exchange-impact on the chemical quality of treated water. Water Res 20:221–232
EPA (2004) Potentiometric determination of nitrate in aqueous samples with ion-selective electrode. Method 9210. Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, SW-846. Available on-line: http://www.epa.gov/ epaoswer/hazwaste/test/9_series.htm
Febrianto J, Kosasih AN, Sunarso J, Ju Y-H, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162:616–645
Ferro S (2003) Removal of nitrates from industrial wastewater: critical reviews. University of Ferrara, Ferrara
Fu F, Dionysiou DD, Liu H (2014) The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J Hazard Mater 267:194–205
Gueye MT, Di Palma L, Allahverdeyeva G, Bavasso I, Petrucci E, Stoller M, Vilardi G (2016) The influence of heavy metals and organic matter on hexavalent chromium reduction by nano zero valent iron in soil. Chem Eng Trans 47:289–294
Guo X, Yang Z, Liu H, Lv X, Tu Q, Ren Q, Xia X, Jing C (2015) Common oxidants activate the reactivity of zero-valent iron (ZVI) and hence remarkably enhance nitrate reduction from water. Sep Purif Technol 146:227–234
Hassani Z, Ghanei Ardekani J, Fallahpour M (2017) Evaluation of environmental impacts of nitrate pollution in Shahre-Kord aquifer and decrease by NZVI,S. Payame Noor University, Department of Taft, M.Sc thesis in environmental geology (In Persian)
Haugen KS, Semmens MJ, Novak PJ (2002) A novel in-situ technology for the treatment of nitrate contaminated groundwater. Water Res 36:3497–3506
Hell F, Lahnsteiner J, Frischherz H, Baumgartner G (1998) Experience with full-scale electrodialysis for nitrate and hardness removal. Desalination 117:173–180
Hiroyuki Y, Tokumura M, Kawase Y (2014) Simultaneous removal of nitrate, hydrogen peroxide and phosphate in semiconductor acidic wastewater by zero-valent iron. J Environ Sci Health A 49(9):998–1006
Hörold S, Vorlop KD, Tacke T, Sell M (1993) Development of catalysts for a selective nitrate and nitrite removal from drinking water. Catal Today 17(1–2):21–30
Hosseini SM (2013) Laboratorial and numbering study of nitrate removal from Shahre-Kord aquifer by nano scale Iron in the reactant wells systems. Research projection of regional water company of Chahrmahal & Bakhtiari
Huang YH, Zhang TC (2004) Effects of low pH on nitrate reduction by iron powder. Water Res 38:2631–2642
Hunter WJ (2001) Use of vegetable oil in a pilot-scale denitrifying barrier. J Contam Hydrol 53:119–131
Hwang Y-H, Kim D-G, Shin H-S (2011) Mechanism study of nitrate reduction by nano zero valent iron. J Hazard Mater 185:1513–1521
Inglezakis VJ, Poulopoulos SG (2006) Adsorption, ion exchange and catalysis: design of operations and environmental applications. Elsevier, Environmental Chemistry
Jensen BV, Jeannie L (2012) Drinking water treatment for nitrate, technical report 6, addressing nitrate in California’s drinking water. Center of Watershed Sciences, University of California, Davis, pp 1–11
Kalaruban M, Loganathan P, Shim WG, Kandasamy J, Ngo HH, Vigneswaran S (2016) Enhanced removal of nitrate from water using amine-grafted agricultural wastes. Sci Total Environ 565:503–510. https://doi.org/10.1016/j.scitotenv.2016.04.194
Kapoor A, Viraraghavan T (1997) Nitrate removal from drinking water—review. J Environ Eng 123(4):371–380
Kim HH, Seagren EA, Davis AP (2003) Engineered bioretention for removal of nitrate from storm water runoff. Water Environ Res 75:355–367
Kumar M, Chakraborty S (2006) Chemical denitrification of water by zero valent magnesium powder. J Hazard Mater B135:112–121
Lalehzari R, Tabatabaei SH, Yarali N (2009) Research of monthly variation of nitrate in Shahre-Kord plain ground water and mapping with GIS. Iranian Water research Journal (IWRJ) 3(4):9–17
Liu A, Ming M, Ankumah RO (2005) Nitrate contamination in private wells in rural Alabama, United States. Sci Total Environ 346:112–120
Luk GK, Au-Yeung WC (2002) Experimental investigation on the chemical reduction of nitrate from groundwater. Adv Environ Res 6:441–453
Lundberg JO, Feelisch M, Björne H, Jansson EA, Weitzberg E (2006) Cardioprotective effects of vegetables: is nitrate the answer? Nitric Oxide 15:359–362. https://doi.org/10.1016/j.niox.2006.01.013
Majumdar D, Gupta N (2000) Nitrate pollution of groundwater and associated human health disorders. Indian J Environ Health 42:28–39
McKay G (2007) Adsorption of dyestuffs from aqueous solutions with activated carbon I: equilibrium and batch contact-time studies. J Chem Technol Biotechnol 32:759–772
Mirzaei S (2009) Assessment of susceptibility and hazard mapping and GIS modeling of the contamination potential of Shahre-Kord aquifer with two models as drastic and syntax. M.Sc thesis of agricultural college, Shahre-Kord University, Pp170 (In Persian)
Mishra D, Farrell J (2005) Understanding nitrate reactions with zerovalent iron using Tafel analysis and electrochemical impedance spectroscopy. Environ Sci Technol 39:645–650
Nuhoglu A, Pekdemir T, Yildiz E, Keskinler B, Akay G (2002) Drinking water denitrification by a membrane bio reactor. Water Res 36:1155–1166
Nujić M, Milinković D, Habuda-Stanić M (2017) Nitrate removal from water by ion exchange. Croat J Food Sci Technol 9(2):182–186
O’Carroll D, Sleep B, Krol M, Boparai H, Kocur C (2013) Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv Water Resour 51:104–122
Pintar A, Batista J, Levec J (2001) Catalytic denitrification: direct and indirect removal of nitrates from potable water. Catal Today 66:503–510
Prusse U, Vorlop KD (2001) Supported bimetallic palladium catalysts for waterphase nitrate reduction. J Mol Catal A Chem 173:313–328
Rocca CD, Belgiorno V, Meric S (2007) Overview of in-situ applicable nitrate removal processes. Desalination 204:46–62
Schoeman JJ, Steyn A (2003) Nitrate removal with reverse osmosis in a rural area in South Africa. Desalination 155:15–26
Shrimali M, Singh KP (2001) New methods of nitrate removal from water. Environ Pollut 112:351 359
Soares MIM (2000) Biological denitrification of groundwater. Water Air Soil Pollut 123:183–193
Sohn K, Kang SW, Ahn S, Woo M, Yang SK (2006) Fe(0) nanoparticles for nitrate reduction: stability, reactivity and transformation. Environ Sci Technol 40:5514–5519
Sowmya A, Meenakshi S (2014) A novel quaternized chitosan-melamine-glutaraldehyde resin for the removal of nitrate and phosphate anions. Int J Biol Macromol 64:224–232
Su C, Puls RW (2007) Removal of added nitrate in the single, binary, and ternary systems of cotton burr compost, zerovalent iron, and sediment: implications for groundwater nitrate remediation using permeable reactive barriers. Chemosphere 67:1653–1662
Su Y, Adeleye AS, Huang Y, Sun X, Dai C, Zhou X, Zhang Y, Keller AA (2014a) Simultaneous removal of cadmium and nitrate in aqueous media by nanoscale zerovalent iron (nZVI) and Au doped nZVI particles. Water Res J 63:102–111
Su Y, Adeleye AS, Zhou X, Dai C, Zhang W, Keller AA, Zhang Y (2014b) Effects of nitrate on the treatment of lead contaminated groundwater by nanoscale zerovalent iron. J Hazard Mater 280:504–513
Tabatabaei SH, Kholghi M, Yarali N, Lalezari R (2009) Research of feeding impacts of Shahre-Kord aquifer by sewage and nitrate diffusion with MT3D model. Research projection of regional water company of Chahrmahal & Bakhtiari (research number: CHE-8600)
Tamara ML, Butler EC (2004) Effects of iron purity and groundwater characteristics on rates and products in the degradation of carbon tetrachloride by iron metal. Environ Sci Technol 38:1866–1876
Till BA, Weathers LJ, Alvarez PJJ (1998) Fe(0)-supported autotrophic denitrification. Environ Sci Technol 32:634–639
Tratnyek PG, Johnson RL (2006) Nanotechnologies for environmental cleanup. Nanotoday 1(2)
Velizarov S, Crespo JG, Reis MA (2004) Removal of inorganic anions from drinking water supplies by membrane bio/processes. Rev Environ Sci Biotechnol
Vilardi G, Di Palma L (2016) Kinetic study of nitrate removal from aqueous solutions using copper-coated iron nanoparticles. Bull Environ Contam Toxicol 98(3):359–365
Vilardi G, Di Palma L, Verdone N (2017a) Competitive reaction modelling in aqueous systems: the case of contemporary reduction of dichromates and nitrates by nZVI. Chem Eng Trans 60:175–180. https://doi.org/10.3303/CET1760030
Vilardi G, Di Palma L, Verdone N (2017b) The influence of nitrate on the reduction of hexavalent chromium by zerovalent iron nanoparticles in polluted wastewater. Desalin Water Treat 86:252–258
Vilardi G, Di Palma L, Verdone N (2018) Heavy metals adsorption by banana peels micro-powder: equilibrium modeling by non-linear models. Chin J Chem Eng 26:455–464
Wilcox LV (1995) Classification and use of irrigation waters. USA Department of Agriculture, Washington, p 19
Yang GCC, Lee HL (2005) Chemical reduction of nitrate by nanosized iron: kinetics and pathways. Water Res 39:884–894
Acknowledgements
This research was performed at Payam-Noor University, so we are grateful of the research adjutantship of the University. Also, journal editors are thanked for handling the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghanei Ardekani, J., Hassani, Z. Study of the environmental impacts of nitrate pollution and its removal by nanoscale zero-valent iron (NZVI) at the south of Shahre-Kord aquifer (Chaharmahal and Bakhtiari province, Iran). Arab J Geosci 11, 708 (2018). https://doi.org/10.1007/s12517-018-4050-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-018-4050-0