Abstract
Purpose of the Review
To provide an overview of the published data on the value of cardiovascular magnetic resonance (CMR) based T1 and T2 and extracellular volume (ECV) fraction quantification to identify myocardial abnormalities related to anthracycline treatment in patients with cancer.
Recent Findings
In animal models of anthracycline cardiotoxicity, elevations in myocardial T1 and T2 values appear to occur early during treatment followed by ventricular remodeling, persistent elevation in T1, and increase in ECV. These findings suggest early myocardial inflammation/edema followed by myocardial fibrosis and ventricular dysfunction. Similarly in patients receiving cancer therapy, T1 and ECV values increase early after anthracycline therapy. The value of T2 mapping in this setting has not been established. Likewise, adult cancer survivors with or without LV dysfunction have increased ECV values which may be associated with worse diastolic parameters. The value of ECV to identify subclinical cardiomyopathy in pediatric cancer survivors remains controversial.
Summary
Quantitative CMR tissue characterization techniques may have a unique role in identifying the pathophysiology of anthracycline-induced cardiomyopathy and for early detection of myocardial injury. In cancer survivors, these techniques have a potential role in identifying early and subclinical myocardial injury. However, the overall literature on quantitative CMR tissue characterization techniques to detect anthracycline cardiotoxicity is limited.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Hooning MJ, Botma A, Aleman BMP, Baaijens MHA, Bartelink H, Klijn JGM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99(5):365–75.
Mulrooney DA, Yeazel MW, Mertens AC, Mitby P, Stovall M, Donaldson SS, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood cancer Survivor Study cohort. BMJ. 2009;339(b4606):1–11.
Keegan THM, Kushi LH, Li Q, Brunson A, Chawla X, Chew HK, et al. Cardiovascular disease incidence in adolescent and young adult cancer survivors : a retrospective cohort study. J Cancer Surviv. 2018;12:388–97.
Chao C, Xu L, Bhatia S, Cooper R, Brar S, Wong FL, et al. Cardiovascular disease risk pro fi les in survivors of adolescent and young adult ( AYA ) cancer : the Kaiser Permanente AYA Cancer Survivors Study. J Clin Oncol. 2019;34(14).
Thavendiranathan P, Abdel-Qadir H, Fischer HD, Camacho X, Amir E, Austin PC, et al. Breast Cancer therapy-related cardiac dysfunction in adult women treated in routine clinical practice: a population-based cohort study. J Clin Oncol. 2016;34(19):2239–46.
Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer : the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2015;100(19):1368–79.
Abdel-Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, et al. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017;2(1):88–93. https://doi.org/10.1001/jamacardio.2016.3841.
Moslehi J. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457–67.
Thavendiranathan P, Wintersperger BJ, Flamm SD, Marwick TH. Cardiac MRI in the assessment of cardiac injury and toxicity from cancer chemotherapy a systematic review. Circ Cardiovasc Imaging. 2013;6(6):1080–91.
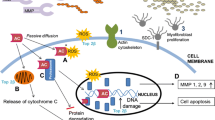
Bernaba BN, Chan JB, Lai CK, Fishbein MC. Pathology of late-onset anthracycline cardiomyopathy. Cardiovasc Pathol. 2010;19(5):308–11. https://doi.org/10.1016/j.carpath.2009.07.004.
Kajihara H, Yasui W, Nakagawa H, Maeda Y. Light and electron microscopic observations of the white pulp of the dog spleen during hemorrhagic shock. Pathol Res Pract. 1986;181(5):586–95 Available from: http://www.sciencedirect.com/science/article/pii/S0344033886801534.
Friedman MA, Bozdech MJ, Billingham ME, Rider AK. Doxorubicin cardiotoxicity: serial endomyocardial biopsies and systolic time intervals. JAMA. 1978;240(15):1603–6. https://doi.org/10.1001/jama.1978.03290150049023.
Bristow MR, Mason JW, Billingham ME, Daniels JR. Doxorubicin cardiomyopathy: evaluation by phonocardiography, endomyocardial biopsy, and cardiac catheterization. Ann Intern Med. 1978;88(2):168–75.
Schelbert EB, Messroghli DR. State of the art: clinical applications of cardiac T1 mapping. Radiology. 2016;278(3):658–76.
Neilan TG, Coelho-Filho OR, Shah RV, Feng JH, Pena-Herrera D, Mandry D, et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol. 2013;111(5):717–22. https://doi.org/10.1016/j.amjcard.2012.11.022.
Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15(1):1–12.
Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, et al. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012;33(10):1268–78.
Reiter G, Reiter C, Kräuter C, Fuchsjäger M, Reiter U. Cardiac magnetic resonance T1 mapping. Part 1: aspects of acquisition and evaluation. Eur J Radiol. 2018;109(October):223–34 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0720048X18303565.
Reiter U, Reiter C, Kräuter C, Fuchsjäger M, Reiter G. Cardiac magnetic resonance T1 mapping. Part 2: diagnostic potential and applications. Eur J Radiol. 2018;109:235–47. https://doi.org/10.1016/j.ejrad.2018.10.013.
Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57(8):891–903. https://doi.org/10.1016/j.jacc.2010.11.013.
Hamlin SA, Henry TS, Little BP, Lerakis S, Stillman AE. Mapping the future of cardiac MR imaging: case-based review of T1 and T2 mapping techniques. RadioGraphics. 2014;34(6):1594–611.
Roujol S, Weingartner S, Foppa M, Chow K, Kawaji K, Ngo LH, et al. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head- to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE1. Radiology. 2014;272(3):683–9.
Messroghli DR, Moon JC, Ferreira VM, Grosse-wortmann L, He T, Kellman P, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1 , T2 , T2 * and extracellular volume : a consensus statement by the Society for Cardiovascular Magnetic Resonance ( SCMR ) endorsed by the European Association for Cardiovascular. J Cardiovasc Magn Reson. 2017;19(75):1–24.
Arheden H, Saeed M, Higgins CB, Gao DW, Bremerich J, Wyttenbach R, et al. Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology. 1999;211(3):698–708.
Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122(2):138–44.
Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 mapping and extracellular volume ( ECV ) in clinical practice : a comprehensive review. J Cardiovasc Magn Reson. 2016;18(89):1–12. https://doi.org/10.1186/s12968-016-0308-4.
Farhad H, Staziaki PV, Addison D, Coelho-Filho OR, Shah RV, Mitchell RN, et al. Characterization of the changes in cardiac structure and function in mice treated with anthracyclines using serial cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2016;9(12):1–8.
Cottin Y, Ribugt C, Maupoil V, Godin D, Arnould L, Brunotte F, et al. Early incidence of adriamycin treatment on cardiac parameters in the rat. Can J Physiol Pharmacol. 2011;72(2):140–5.
Lightfoot JC, D’Agostino RB, Hamilton CA, Jordan J, Torti FM, Kock ND, et al. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. 2010;3(5):550–8.
Hong YJ, Park HS, Park JK, Han K, Park CH, Kim TK, et al. Early detection and serial monitoring of anthracycline-induced cardiotoxicity using T1-mapping cardiac magnetic resonance imaging: an animal study. Sci Rep. 2017;7(1):1–10.
Hong YJ, Kim TK, Hong D, Park CH, Yoo SJ, Wickum ME, et al. Myocardial characterization using dual-energy CT in doxorubicin-induced DCM: comparison with CMR T1-mapping and histology in a rabbit model. JACC Cardiovasc Imaging. 2016;9(7):836–45.
• Galán-Arriola C, Lobo M, Vílchez-Tschischke JP, López GJ, de Molina-Iracheta A, Pérez-Martínez C, et al. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J Am Coll Cardiol. 2019;73(7):779–91 Recent original research paper in an animal model that shows T2 mapping as the earliest marker of cardiotoxicity in doxorubicin-treated pigs, and in the absence of other altered CMR parameters, proposing a reversible disease stage.
Meléndez GC, Jordan JH, D’Agostino RB, Vasu S, Hamilton CA, Hundley WG. Progressive 3-month increase in LV myocardial ECV after anthracycline-based chemotherapy. JACC Cardiovasc Imaging. 2017;10(6):708–9. https://doi.org/10.1016/j.jcmg.2016.06.006.
Muehlberg F, Funk S, Zange L, von Knobelsdorff-Brenkenhoff F, Blaszczyk E, Schulz A, et al. Native myocardial T1 time can predict development of subsequent anthracycline-induced cardiomyopathy. ESC Hear Fail. 2018;5(4):620–9.
• Haslbauer JD, Lindner S, Valbuena-Lopez S, Zainal H, Zhou H, D’Angelo T, et al. CMR imaging biosignature of cardiac involvement due to cancer-related treatment by T1 and T2 mapping. Int J Cardiol. 2019;275:179–86 This paper found a positive correlation between native T1 and T2 values and high sensitive troponin, and between native T1 values, reduced strain and increased end-systolic volumes, proposing phenotypical signatures for early (inflammatory) and late (fibrotic) cardiac involvement.
• Ferreira de Souza T, Quinaglia AC, Silva T, Osorio Costa F, Shah R, Neilan TG, et al. Anthracycline therapy is associated with cardiomyocyte atrophy and preclinical manifestations of heart disease. JACC Cardiovasc Imaging. 2018;11(8):1045–55 This paper proposes total LV cardiomyocyte mass and intracellular water lifetime as new CMR parameters to evaluate for cardiomyocyte atrophy, suggesting that an increase in ECV can be explained by myocyte loss or by a decrease in myocyte size. The decrease in cardiomyocyte mass was higher in patients with elevated cardiac troponin T levels, suggesting a higher degree of cardiomyocyte injury.
Takagi H, Ota H, Umezawa R, Kimura T, Kadoya N, Higuchi S, et al. Left ventricular T1 mapping during chemotherapy – radiation therapy : serial assessment of participants with esophageal cancer. Radiology. 2018;289(2):347–54. https://doi.org/10.1148/radiol.2018172076.
Tong X, Li VW, Liu AP, So EK, Chan Q, Ho KK, et al. Cardiac magnetic resonance T1 mapping in adolescent and young adult survivors of childhood cancers. Circ Cardiovasc Imaging. 2019;12(4):1–3 Available from: https://www.ahajournals.org/doi/10.1161/CIRCIMAGING.118.008453.
Kimball A, Patil S, Koczwara B, Raman KS, Perry R, Grover S, et al. Late characterisation of cardiac effects following anthracycline and trastuzumab treatment in breast cancer patients. Int J Cardiol. 2018;261:159–61. https://doi.org/10.1016/j.ijcard.2018.03.025.
• Jordan JH, Vasu S, Morgan TM, D’Agostino RB, Meléndez GC, Hamilton CA, et al. Anthracycline-associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging. 2016;9(8):1–9 In this study with long-term follow-up of cancer survivors, ECV remained abnormally elevated in patients after 3 years of recieving anthracycline-based chemotherapy, and coincided with a decrease in left ventricular mass and function over time.
Tham EB, Haykowsky MJ, Chow K, Spavor M, Kaneko S, Khoo NS, et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15(1):48 Available from: http://jcmr-online.biomedcentral.com/articles/10.1186/1532-429X-15-48.
Toro-Salazar OH, Gillan E, O’Loughlin MT, Burke GS, Ferranti J, Stainsby J, et al. Occult cardiotoxicity in childhood cancer survivors exposed to anthracycline therapy. Circ Cardiovasc Imaging. 2013;6(6):873–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-Oncology
Rights and permissions
About this article
Cite this article
Urzua Fresno, C., Shalmon, T., Calvillo Argüelles, O. et al. Cardiovascular Magnetic Resonance Relaxometry in Early Detection of Anthracycline Cardiotoxicity. Curr Cardiovasc Imaging Rep 13, 2 (2020). https://doi.org/10.1007/s12410-019-9524-2
Published:
DOI: https://doi.org/10.1007/s12410-019-9524-2