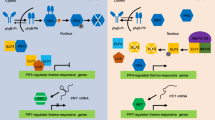
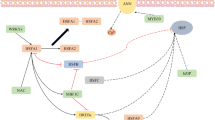
Abstract
Plants are capable of actively adapting to a variety of environmental signals by adjusting physiological activities, growth behaviors, and architectural morphology. Temperatures are one of the major environmental factors that extensively affect plant growth and developmental processes. A suite of signaling mediators and underlying molecular mechanisms governing plant adaptation to stressful high and low temperatures have been explored through molecular genetic approaches and global-scale gene expression screening in recent decades. Notably, it is known that ambient temperature changes even by a few degrees also profoundly affect plant developmental and morphological traits. In particular, in response to ambient warm temperatures, plants exhibit distinct morphological adjustments, such as stem elongation, increase of leaf hyponasty, and formation of small, slender leaves, which are collectively termed thermomorphogenesis. Plant thermomorphogenesis is intimately associated with global warming, which represents a steady increase of the average global temperatures and is emerging as a world-wide ecological concern because of its impacts on plant vegetation and crop productivity. In this minireview, we summarize recent progress in the field and discuss practical insights into how plants cope with temperature changes to sustain optimal growth and adaptive behaviors.



Similar content being viewed by others
References
Alcázar R, Parker JE (2011) The impact of temperature on balancing immune responsiveness and growth in Arabidopsis. Trends Plant Sci 12:666–675
Begum S, Nakaba S, Bayramzadeh V, Oribe Y, Kubo T, Funada R (2008) Temperature responses of cambial reactivation and xylem differentiation in hybrid poplar (Populus sieboldii x P. grandidentata) under natural conditions. Tree Physiol 28(12):1813–1819
Begum S, Nakaba S, Oribe Y, Kubo T, Funada R (2010) Cambial sensitivity to rising temperatures by natural condition and artificial heating from late winter to early spring in the evergreen conifer Cryptomeria japonica. Trees 24(1):43–52
Bose BK (2010) Global warming: energy, environmental pollution, and the impact of power electronics. IEEE Ind Electron Mag 4:6–17
Braslavsky SE, Gärtner W, Schaffner K (1997) Phytochrome photoconversion. Plant Cell Environ 20:700–706
Castillon A, Shen H, Huq E (2007) Phytochrome interacting factors: central players in phytochrome-mediated light signaling networks. Trends Plant Sci 12:514–521
Chakraborty S, Newton AC (2011) Climate change, plant diseases and food security: an overview. Plant Pathol 60:2–14
Chaneva S, Sevdalina F, Minkova K, Lukavský J (2007) Effect of light and temperature on the cyanobacterium Arthronema africanum—a prospective phycobiliprotein-producing strain. J Appl Phycol 19(5):537–544
Cobben MMP, Verboom J, Opdam PFM, Hoekstra RF, Jochem R, Arens P, Smulders MJM (2011) Projected climate change causes loss and redistribution of genetic diversity in a model metapopulation of a medium-good disperser. Ecography 34:920–932
Commuri PD, Jones RD (2001) High temperatures during endosperm cell division in maize: a genotypic comparison under in vitro and field conditions. Crop Sci 41:1122–1130
Deutsch CA, Tewksbury JJ, Tigchelaar M, Battisti DS, Merrill SC, Huey RB, Naylor RL (2018) Increase in crop losses to insect pests in a warming climate. Science 361:916–919
Dong CH, Agarwal M, Zhang Y, Xie Q, Zhu JK (2006) The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc Natl Acad Sci USA 103:8281–8286
Elich TD, Chory J (1997) Biochemical characterization of Arabidopsis wild-type and mutant phytochrome B holoproteins. Plant Cell 9:2271–2280
Fei Q, Li J, Luo Y, Ma K, Niu B, Mu C, Gao H, Li X (2018) Plant molecular responses to the elevated ambient temperatures expected under global climate change. Plant Signal Behav 13:e1414123
Fitter AH, Fitter RS (2002) Rapid changes in flowering time in British plants. Science 296:1689–1691
Franklin KA, Lee SH, Patel D, Kumar SV, Spartz AK, Gu C, Ye S, Yu P, Breen G, Cohen JD, Wigge PA, Gray WM (2011) Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc Natl Acad Sci USA 108:20231–20235
Fujii Y, Tanaka H, Konno N, Ogasawara Y, Hamashima N, Tamura S, Hasegawa S, Hayasaki Y, Okajima K, Kodama Y (2017) Phototropin perceives temperature based on the lifetime of its photoactivated state. Proc Natl Acad Sci USA 114(34):9206–9211
Gangappa SN, Kumar SV (2017) DET1 and HY5 control PIF4-mediated thermosensory elongation growth through distinct mechanisms. Cell Rep 18(2):344–351
Gangappa SN, Berriri S, Kumar SV (2017) PIF4 coordinates thermosensory growth and immunity in Arabidopsis. Curr Biol 27:243–249
Gil KE, Kim WY, Lee HJ, Faisal M, Saquib Q, Alatar AA, Park CM (2017) ZEITLUPE contributes to a thermoresponsive protein quality control system in Arabidopsis. Plant Cell 29:2882–2894
Gould PD, Locke JC, Larue C, Southern MM, Davis SJ, Hanano S, Moyle R, Milich R, Putterill J, Millar AJ, Hall A (2006) The molecular basis of temperature compensation in the Arabidopsis circadian clock. Plant Cell 18:1177–1187
Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M (1998) High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA 95:7197–7202
Ha JH, Han SH, Lee HJ, Park CM (2017a) Environmental adaptation of the heterotrophic-to-autotrophic transition: the developmental plasticity of seedling establishment. Crit Rev Plant Sci 36:128–137
Ha JH, Lee HJ, Jung JH, Park CM (2017b) Thermo-induced maintenance of photo-oxidoreductases underlies plant autotrophic development. Dev Cell 41:170–179
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684
Holmgren M, Scheffer M, Huston MA (1997) The interplay of facilitation and competition in plant communities. Ecology 78:1966–1975
Huey RB, Carlson M, Crozier L, Frazier M, Hamilton H, Harley C, Hoang A, Kingsolver JG (2002) Plants versus animals: do they deal with stress in different ways? Integr Comp Biol 42:415–423
Huot B, Castroverde CDM, Velásquez AC, Hubbard E, Pulman JA, Yoshida Y, Yao J, Howe GA, Childs KL, Tsuda K, Montgomery BL, He SY (2017) Dual impact of elevated temperature on plant defence and bacterial virulence in Arabidopsis. Nat Commun 8:1808
Huq E (2002) Quail PH (2002) PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J 21:2441–2450
James R, Washington R (2013) Changes in African temperature and precipitation associated with degrees of global warming. Clim Change 117:859–872
Jump AS, Marchant R, Peñuelas J (2009) Environmental change and the option value of genetic diversity. Trends Plant Sci 14:51–58
Jung JH, Seo PJ, Ahn JH, Park CM (2012) Arabidopsis RNA-binding protein FCA regulates microRNA172 processing in thermosensory flowering. J Biol Chem 287:16007–16016
Jung JH, Domijan M, Klose C, Biswas S, Ezer D, Gao M, Khattak AK, Box MS, Charoensawan V, Cortijo S, Kumar M, Grant A, Locke JC, Schäfer E, Jaeger KE, Wigge PA (2016) Phytochromes function as thermosensors in Arabidopsis. Science 354:886–889
Kelly AE, Goulden ML (2008) Rapid shifts in plant distribution with recent climate change. Proc Natl Acad Sci USA 105:11823–11826
Kim HY, Horie T, Nakagawa H, Wada K (1996) Effects of elevated CO2 concentration and high temperature on growth and yield of rice: II. The effect of yield and its component of Akihikari rice. Jpn J Crop Sci 65:644–651
Kim J, Yi H, Choi G, Shin B, Song PS, Choi G (2003) Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction. Plant Cell 15:2399–2407
Kim TS, Kim WY, Fujiwara S, Kim J, Cha JY, Park JH, Lee SY, Somers DE (2011) HSP90 functions in the circadian clock through stabilization of the client F-box protein ZEITLUPE. Proc Natl Acad Sci USA 108:16843–16848
Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, Franklin KA (2009) High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol 19:408–413
Łabuz J, Hermanowicz P, Gabryś H (2015) The impact of temperature on blue light induced chloroplast movements in Arabidopsis thaliana. Plant Sci 239:238–249
Lee HJ, Jung JH, Cortés Llorca L, Kim SG, Lee S, Baldwin IT, Park CM (2014) FCA mediates thermal adaptation of stem growth by attenuating auxin action in Arabidopsis. Nat Commun 5:5473
Lee HJ, Ha JH, Park CM (2017) An FCA-mediated epigenetic route towards thermal adaptation of autotrophic development in plants. BMB Rep 50:343–344
Legris M, Klose C, Burgie ES, Rojas CC, Neme M, Hiltbrunner A, Wigge PA, Schäfer E, Vierstra RD, Casal JJ (2016) Phytochrome B integrates light and temperature signals in Arabidopsis. Science 354:897–900
Más P, Kim WY, Somers DE, Kay SA (2003) Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature 426:567–570
Mathews S (2010) Evolutionary studies illuminate the structural-functional model of plant phytochromes. Plant Cell 22(1):4–16
Matsuzaki J, Kawahara Y, Izawa T (2015) Punctual transcriptional regulation by the rice circadian clock under fluctuating field conditions. Plant Cell 27:633–648
Mattoo RU, Sharma SK, Priya S, Finka A, Goloubinoff P (2013) Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates. J Biol Chem 19:21399–21411
Maurya JP, Bhalerao RP (2017) Photoperiod- and temperature-mediated control of growth cessation and dormancy in trees: a molecular perspective. Ann Bot 120:351–360
McClellan AJ, Tam S, Kaganovich D, Frydman J (2005) Protein quality control: chaperones culling corrupt conformations. Nat Cell Biol 7:736–741
Medzihradszky M, Bindics J, Ádám É, Viczián A, Klement É, Lorrain S, Gyula P, Mérai Z, Fankhauser C, Medzihradszky KF, Kunkel T, Schäfer E, Nagy F (2013) Phosphorylation of phytochrome B inhibits light-induced signaling via accelerated dark reversion in Arabidopsis. Plant Cell 25:535–544
Meehl GA, Washington WM, Collins WD, Arblaster JM, Hu A, Buja LE, Strand WG, Teng H (2005) How much more global warming and sea level rise? Science 307:1769–1772
Menna A, Nguyen D, Guttman DS, Desveaux D (2015) Elevated temperature differentially influences effector-triggered immunity outputs in Arabidopsis. Front Plant Sci 6:995
Miura K, Furumoto T (2013) Cold signaling and cold response in plants. Int J Mol Sci 14:5312–5337
Mooney KA, Halitschke R, Kessler A, Agrawal AA (2010) Evolutionary trade-offs in plants mediate the strength of trophic cascades. Science 327:1642–1644
Oh E, Kim J, Park E, Kim JI, Kang C, Choi G (2004) PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16:3045–3058
Ortigosa A, Fonseca S, Franco-Zorrilla JM, Fernandez-Calvo P, Zander M, Lewsey MG, García-Casado G, Fernández-Barbero G, Ecker JR, Solano R (2019) The JA-pathway MYC transcription factors regulate photomorphogenic responses by targeting HY5 gene expression. Plant J. https://doi.org/10.1111/tpj.14618
Park YJ, Park CM (2019) Physicochemical modeling of the phytochrome-mediated photothermal sensing. Sci Rep 9:10485
Park YJ, Lee HJ, Ha JH, Kim JY, Park CM (2017) COP1 conveys warm temperature information to hypocotyl thermomorphogenesis. New Phytol 215:269–280
Park YJ, Lee HJ, Gil KE, Kim JY, Lee JH, Lee H, Cho HT, Vu LD, De Smet I, Park CM (2019) Developmental programming of thermonastic leaf movement. Plant Physiol 180:1185–1197
Pyl ET, Piques M, Ivakov A, Schulze W, Ishihara H, Stitt M, Sulpice R (2012) Metabolism and growth in Arabidopsis depend on the daytime temperature but are temperature-compensated against cool nights. Plant Cell 24:2443–2469
Rockwell NC, Lagarias JC (2006) The structure of phytochrome: a picture is worth a thousand spectra. Plant Cell 18:4–14
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Scharf KD, Höhfeld I, Nover L (1998) Heat stress response and heat stress transcription factors. J Biosci 23:313–329
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
Velásquez AC, Castroverde CDM, He SY (2018) Plant and pathogen warfare under changing climate conditions. Curr Biol 28:R619–R634
Xiao S, Brown S, Patrick E, Brearley C, Turner JG (2003) Enhanced transcription of the Arabidopsis disease resistance genes RPW8.1 and RPW8.2 via a salicylic acid-dependent amplification circuit is required for hypersensitive cell death. Plant Cell 15(1):33–45
Zeng N, Yoon J (2009) Expansion of the world's deserts due to vegetation-albedo feedback under global warming. Grophys Res 36:L17401
Zhu J, Dong CH, Zhu JK (2007) Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Curr Opin Plant Biol 10:290–295
Acknowledgements
This work was supported by the Leaping Research (NRF-2018R1A2A1A19020840) Program provided by the National Research Foundation of Korea (NRF) and the Next-Generation BioGreen 21 Program (PJ013134) provided by the Rural Development Administration of Korea. Y.-J.P. was partially supported by Global Ph.D. Fellowship Program through NRF (NRF-2016H1A2A1906534).
Author information
Authors and Affiliations
Contributions
CMP and YJP designed the manuscript. CMP, YJP, JHL, and JYK wrote the manuscript. J.-I Kim provided scientific discussion. All the authors agreed on the contents of the paper and critically evaluated the manuscript.
Corresponding authors
Rights and permissions
About this article
Cite this article
Lee, JH., Kim, J.Y., Kim, JI. et al. Plant Thermomorphogenic Adaptation to Global Warming. J. Plant Biol. 63, 1–9 (2020). https://doi.org/10.1007/s12374-020-09232-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-020-09232-y